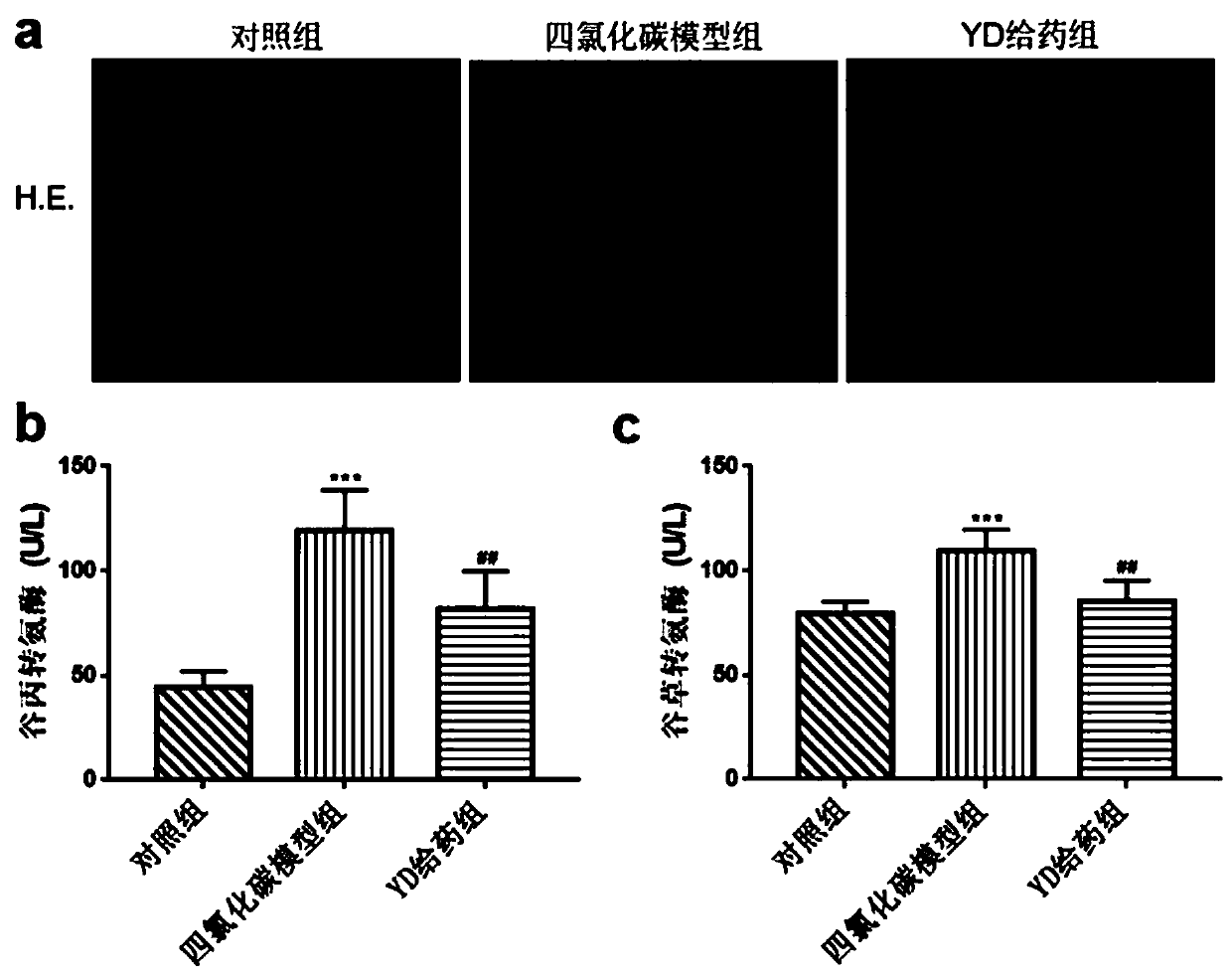
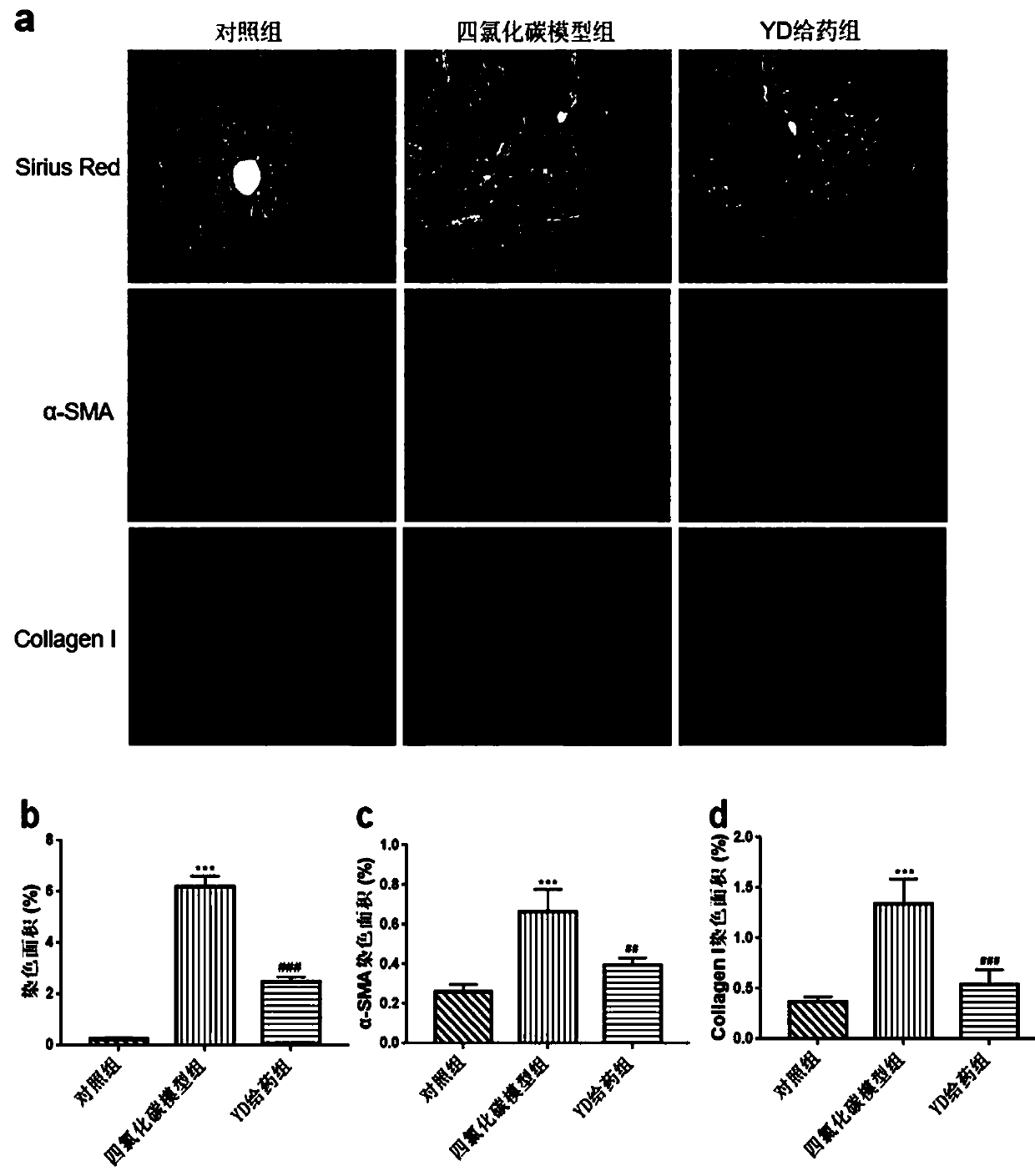
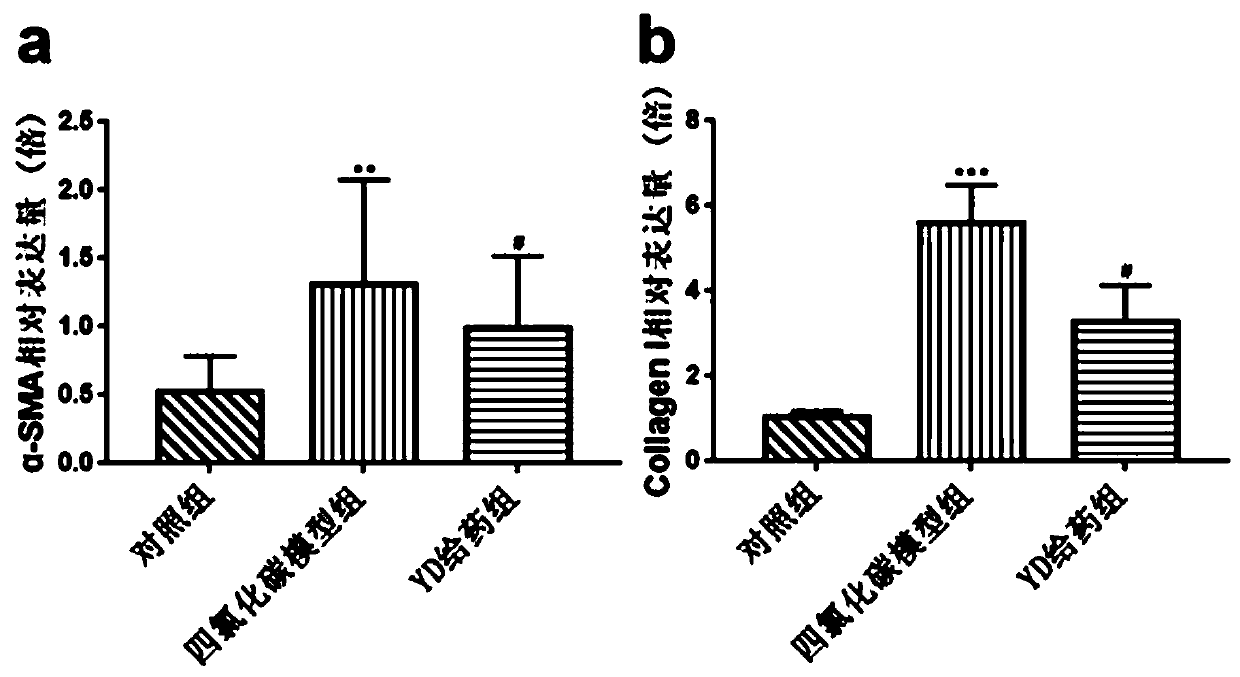
Application of antibacterial peptide YD in preparing drug for treating hepatic fibrosis
A technology of liver fibrosis and antimicrobial peptides, applied in the field of biomedicine, can solve the problems of no reports of antimicrobial peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Preparation of antimicrobial peptide YD freeze-dried powder for intramuscular injection
[0106] Formula: antibacterial peptide YD (purity greater than 95%) 30.0g
[0107] Specific protein stabilizer, lyoprotectant / excipient 30.0g
[0108] Osmolality regulator (plasma 313 mOsm / kgH 2 O) 0.5-1L
[0109] pH regulator (pH 4-9) 0.5-1L
[0110] Pharmaceutical water Add to total volume 60L
[0111] The total mass of freeze-dried powder is 60.0-70.0g
[0112] Process: According to the conventional process for preparing freeze-dried powder, a total of 1,000 freeze-dried powders were prepared, each containing 60-70 mg of antimicrobial peptide YD freeze-dried powder, and stored away from light.
[0113] The above-mentioned non-specific protein stabilizer and lyoprotectant are preferably mannitol, the excipient is preferably lactose, the osmotic pressure regulator is preferably NaCl, and the pH regulator is preferably citric acid / Tris-HCl buffer.
Embodiment 2
[0115] Preparation of Oral Preparation of Antimicrobial Peptide YD
[0116] Formula: antibacterial peptide YD (purity greater than 95%) 50.0g
[0117] Filler 85.0g
[0118] Adhesive 5.0g
[0119] Lubricant 10.0g
[0120] Total 200.0g
[0121] Process: 1,000 capsules are made according to the conventional process for preparing capsules, each capsule contains 50 mg of the active ingredient of antibacterial peptide YD, and the capsules are coated with an enteric-coated material acrylic resin to make an enteric-coated preparation. Excipients such as fillers, adhesives, and lubricants are conventional excipients in pharmacy.
[0122] In addition, oral preparations can also be made of microspheres, microcapsules, and nanospheres by wrapping the above-mentioned fillers, binders, lubricants and other auxiliary materials with polyester materials, and liposomes or nanospheres by wrapping chitosan. Granules, adding cholesterol, phospholipids and free fatty acids to prepare microemul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com