Porcine circovirus type 2/mycoplasma hyorhinis combined vaccine
A technology of porcine circovirus and mycoplasma hyorhina, which is applied in the field of porcine vaccines and can solve the problems of vaccine products without mycoplasma hyorhina
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
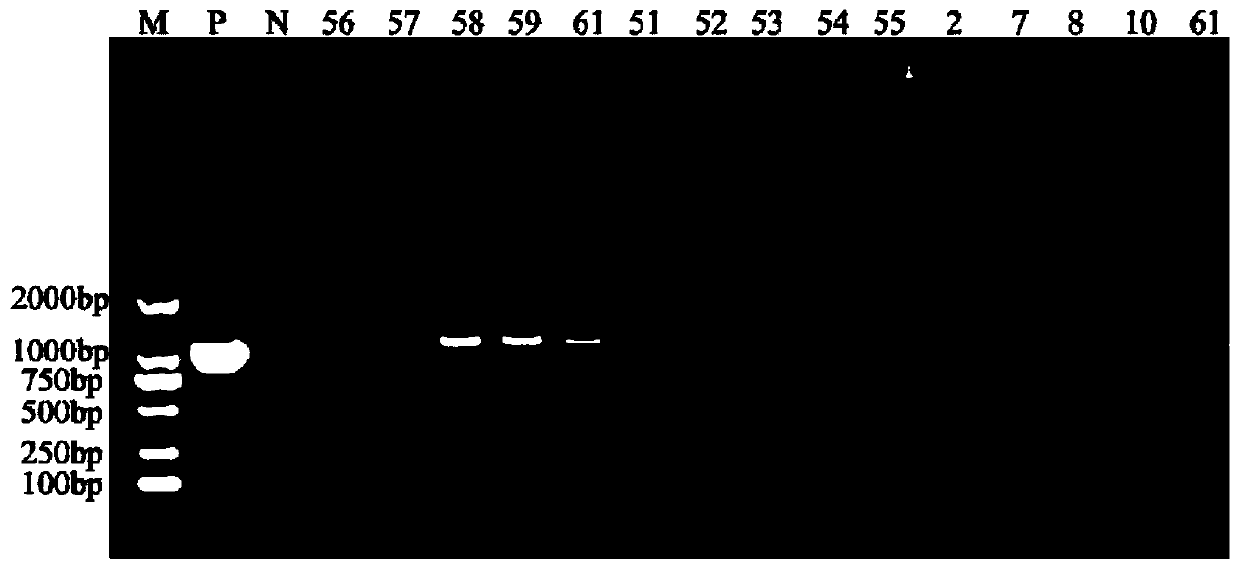
Image
Examples
Embodiment 1
[0027] The preparation of embodiment 1 porcine circovirus type 2 (PCV2) antigen
[0028] Viruses, Cells, Antibodies and Reagents:
[0029] Porcine circovirus type 2 LG strain was isolated from sick pigs with clinical manifestations of PMWS. PK15 cells were identified as being free from porcine circovirus type 2 and type 1 contamination. The standard positive serum of porcine circovirus type 2 LG strain was obtained from Harbin, China Preserved by the Institute of Veterinary Medicine.
[0030] Porcine circovirus type 2 LG strain culture:
[0031] 1. Full suspension culture of PK15 cells
[0032] 1.1 Optimization of the initial seeding density of cells Adjust the seeding density of PK15 cells to 10×10 3 / ml, 10×10 4 / ml, 30×10 4 / ml, 50×10 4 / ml, 80×10 4 / ml and 10×10 5 / ml, respectively in the bioreactor for full suspension culture (medium from Shanghai Yuanpei Biotechnology Co., Ltd. serum-free full suspension medium). The results showed that even if the cell seeding ...
Embodiment 2
[0068] The preparation of embodiment 2 mycoplasma hyorhina antigen
[0069] Viruses, cells, antibodies and reagents: Mycoplasma hyorhina DL strain was isolated from sick pigs with clinical manifestations of PMWS, and the standard positive serum of Mycoplasma hyorhina DL strain was preserved by Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences.
[0070] Culture of Mycoplasma hyorhina: Use the 10th generation culture of Mycoplasma hyorhina DL strain as the virus seed, inoculate it into the in vitro culture medium of Mycoplasma hyorhina synchronously (conventional substances such as PPLO broth, hydrolyzed milk protein and glucose) according to the dose of 0.2%, and place it at 37°C Shake culture under culture conditions for 40 h to harvest, measure CCU, centrifuge at 8000 rpm for 15 min, discard the supernatant and save the precipitate for later use.
[0071] Inactivation of Mycoplasma hyorhina: Recover the centrifugally purified Mycoplasma hyorhina p...
Embodiment 3
[0072] Example 3 Preparation of porcine circovirus type 2 / mycoplasma hyorhina dual vaccine
[0073] Utilize the water-based adjuvant according to the manufacturer's instruction method (the ratio of the mineral oil adjuvant to the water-phase antigen is 1:10, and the oil phase is slowly added to the water-phase antigen (porcine circovirus prepared respectively in Examples 1 and 2) 2 and Mycoplasma hyorhina), stirring at low shear for 30 minutes) to prepare the dual vaccine of porcine circovirus type 2 and Mycoplasma hyorhina (LG strain+DL strain). The sample of porcine circovirus type 2 / mycoplasma hyorhina dual vaccine was prepared, and the physical properties of the vaccine were tested, showing that the stability, viscosity, appearance and sterility test of the vaccine all met the requirements.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com