Crystalline form III of oxazolidinone antibacterial drug free acid and preparation method and application of crystalline form III of oxazolidinone antibacterial drug free acid
A technology for antibacterial drugs and oxazolidinones, applied in the field of medicine, can solve the problem of high crystallinity, achieve good stability, significant economic and social benefits, and improve the effects of medicinal value and commercial value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
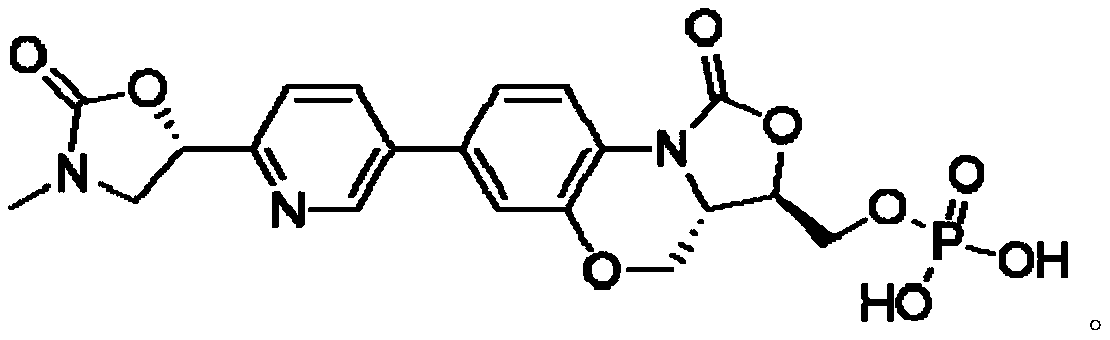
[0020] In the specific implementation of the present invention, a method for preparing the crystal form III of the free acid of oxazolidinone antibacterial drugs is that the reaction process formula is:
[0021]
[0022] According to the above formula, a method for preparing the crystal form III of the free acid of an oxazolidinone antibacterial drug of the present invention comprises the following steps:
[0023] (1), add 100g of free acid precursor molecules to 4L of 1,4-dioxane to form a solution, then add the solution to 6M 30 equivalent hydrochloric acid solution at 0-5°C to form Reaction mixture; heat the reaction mixture to 55°C, stir at room temperature for 10 hours, then cool to 0-5°C, adjust the pH value to 2-3 with 1M 1 equivalent of NaOH, continue to stir at 0-5°C for 1 hour, filter , the filter cake was washed at 10-20° C. for 2 hours with 10 times the volume of methanol, filtered, and dried to obtain 89 g of oxazolidinone antibacterial drug free acids shown in...
Embodiment 2
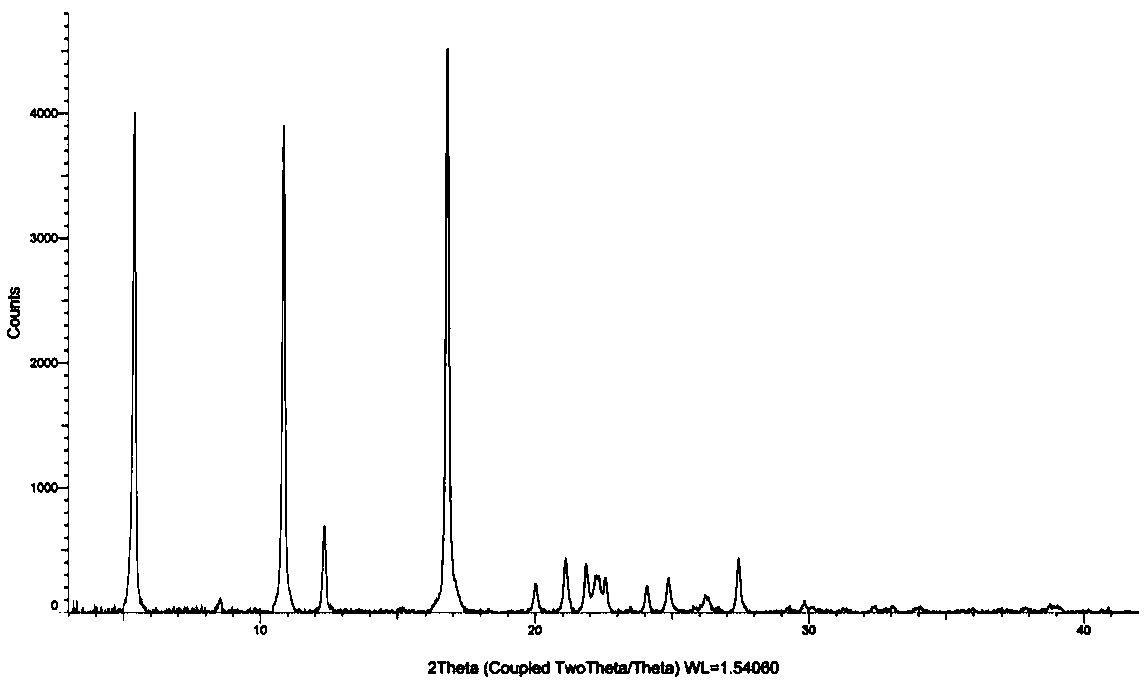
[0026] A method for preparing crystal form III of oxazolidinone antibacterial drug free acid of the present invention, take 10g of the oxazolidinone antibacterial drug free acid free acid prepared in embodiment 1 step (1) without fixed solid, add water 200mL, Stir at 40°C for 60 hours, filter, and vacuum-dry the filter cake at 50°C for 28 hours to obtain 8.8 g of crystal form III solid with a yield of 88%. The obtained solid is subjected to powder X-ray diffraction, and its X-ray diffraction pattern is as follows: figure 1 shown.
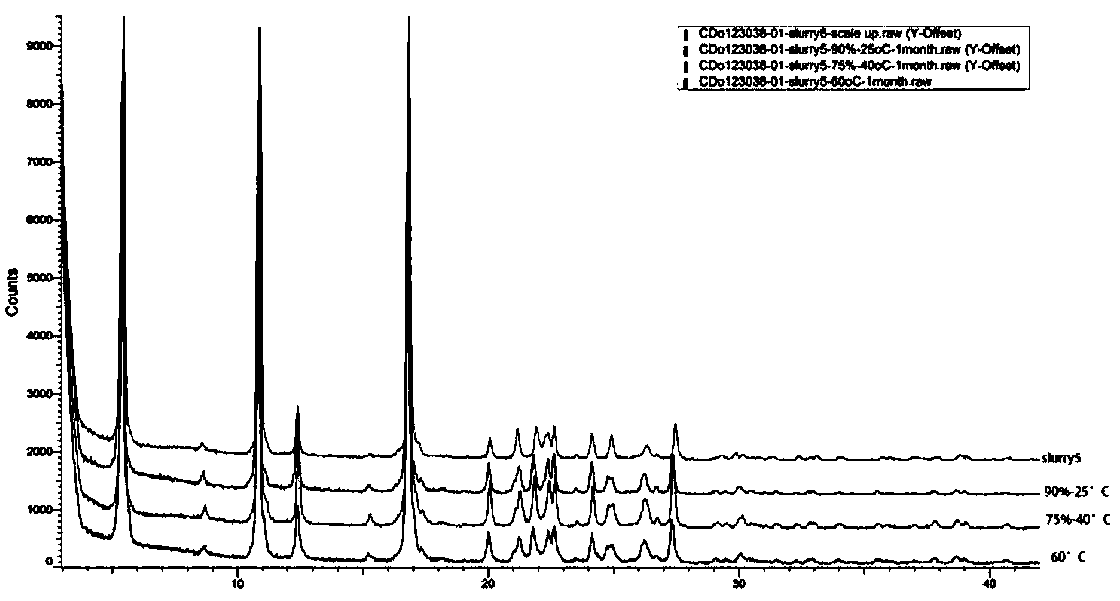
[0027] The crystal form III prepared in Example 1 and Example 2 was determined by X-ray diffraction and contained the following characteristic peaks: 5.4±0.2°, 8.6±0.2°, 10.9±0.2°, 12.4±0.2°, 16.8± 0.2°, 20.0±0.2°, 21.1±0.2°, 21.9±0.2°, 22.3±0.2°, 22.6±0.2°, 24.1±0.2°, 24.9±0.2°, 26.2±0.2°, 27.4±0.2°, 29.8± 0.2°, this crystal form has high crystallinity and good stability in aqueous solution. It is suitable for the preparation of pharmaceutical pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com