Preparation method and application of a novel pt(iv) complex with tumor targeting
A tumor-targeting and complex technology, applied in platinum-based organic compounds, platinum-group organic compounds, anti-tumor drugs, etc., can solve the problems of difficult combined chemotherapy regimens, low activity, and different mechanisms of action, so as to avoid drug resistance Drug properties, simple and easy operation, beneficial to the effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Embodiment 1: prepare complex (A-1) as shown in I formula, namely:
[0086]
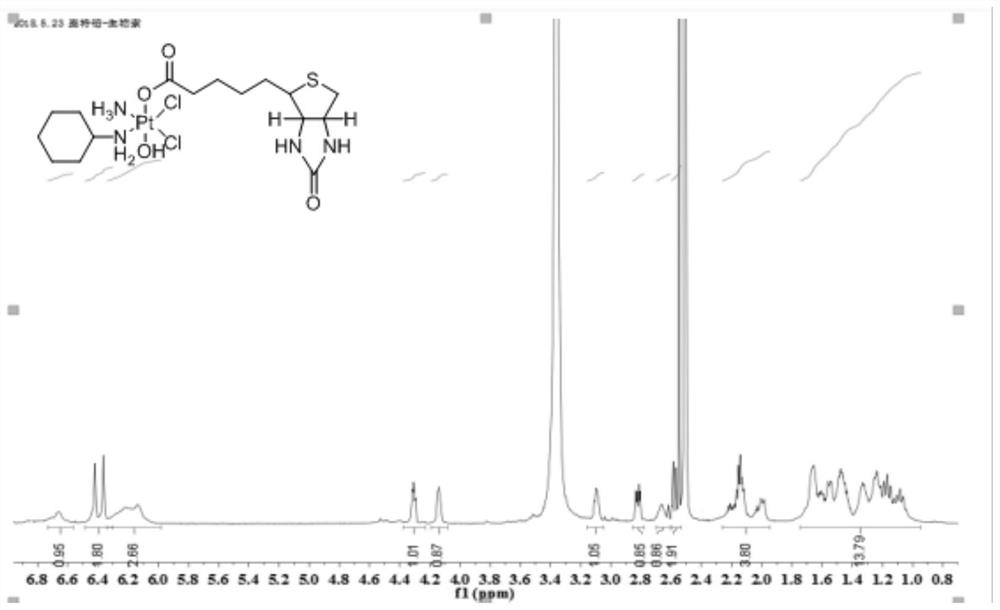
[0087] Weigh 1 g (1.6 mol) of the Pt(IV) complex precursor containing two hydroxyl groups prepared in step 5, dissolve it in an appropriate amount of DMSO, and place it in a 100 mL round bottom flask. Weigh 0.95 g (1.6 mol) of the biotin succinimide ester prepared in step 9 and add it to the above reaction solution, put it in a water bath at 60°C, and stir evenly overnight. After the reaction was finished, a small amount of undissolved solid in the reaction solution was removed by suction filtration, and the filtrate was collected. The filtrate was recrystallized with diethyl ether and methanol at a volume ratio of 1:1 to precipitate the product, filtered with suction, rinsed with the mother liquor 2 to 3 times, and dried in vacuum to obtain a Pt (IV) Complex 0.4g, its hydrogen spectrum is as follows figure 1 As shown, its mass spectrum is shown as figure 2 shown. Yield: 26.7%. 1 H NM...
Embodiment 2
[0088] Embodiment 2: prepare complex (A-2) as shown in I formula, namely:
[0089]
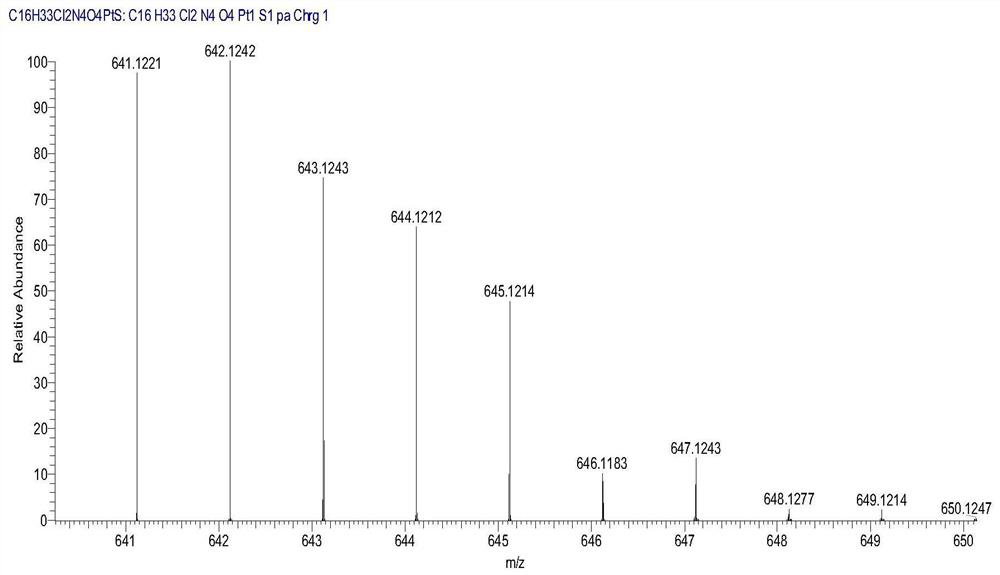
[0090] Weigh the Pt(IV) complex precursor 1g (1.12mol) containing monochlorine and monohydroxyl prepared in step 7 and dissolve it in an appropriate amount of DMF solvent, then add condensing agent TBTU 1.1g (1.12mol) and triethylamine 0.24 g (1mol), add to the reaction system, and stir evenly in a water bath at 40°C. Weigh 0.526g (0.499mol) of biotin and add it to the reaction solution, and continue the reaction overnight. After the reaction was finished, filter with suction to remove a small amount of undissolved solid in the reaction solution, and collect the filtrate. Add appropriate amount of CH to the reaction solution 2 Cl 2 Extract with purified water for 3 times to remove the condensing agent in the reaction solution, collect the organic phase liquid, evaporate and concentrate to remove the organic solvent, the product is recrystallized with ether and methanol with a volume rati...
Embodiment 3
[0091] Embodiment 3: prepare complex (A-3) as shown in I formula, namely:
[0092]
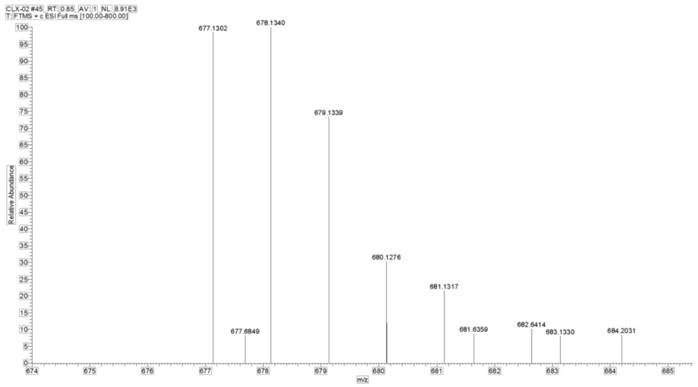
[0093]Weigh 1 g (1.6 mol) of the Pt(IV) complex precursor containing two hydroxyl groups prepared in step 6, dissolve it in an appropriate amount of DMSO, and place it in a 100 mL round bottom flask. Weigh 0.95 g (1.6 mol) of the biotin succinimide ester prepared in step (9) and add it to the above reaction solution, place in a water bath at 60°C, and stir evenly overnight. After the reaction was finished, a small amount of undissolved solid in the reaction solution was removed by suction filtration, and the filtrate was collected. The filtrate was recrystallized with diethyl ether and methanol at a volume ratio of 1:1 to precipitate the product, filtered with suction, rinsed with the mother liquor 2 to 3 times, and dried in vacuum to obtain a Pt (IV) complex 0.48g, its hydrogen spectrogram is as Figure 5 As shown, its mass spectrum is as Figure 6 shown. Yield: 32.00%. 1 H NMR (600MH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com