Novel anti-tumor Pt(IV) complex capable of being orally taken, and preparation method and application thereof
An anti-tumor and complex technology, applied in the field of the preparation of new anti-tumor Pt complexes, can solve the problems of complex synthesis process of JM216, difficult combination chemotherapy, high treatment cost, avoid drug resistance, and the synthesis process is simple and easy to operate. , the effect of simple and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1: Preparation of compound (C-1) shown in formula II, namely:
[0067]
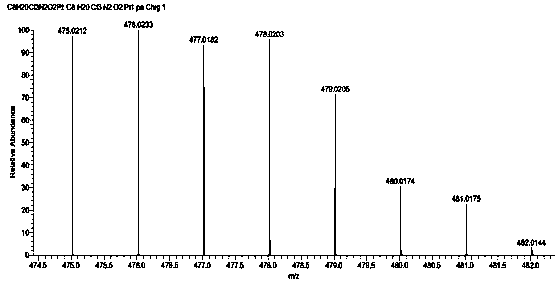
[0068] Dissolve 1.5g (1.1mol) of the Pt(II) compound prepared in step 3 in 20mL of acetic acid, weigh 0.524g (1.1mol) of N-chlorosuccinimide and add it to the reaction system, and stir evenly at room temperature overnight . After the reaction was completed, 66 was evaporated and concentrated under reduced pressure to remove the solvent, and dried to obtain 0.45 g of a Pt(IV) complex with a lipophilic group in the axial direction. Yield: 24.051%. Its hydrogen spectrum is as figure 1 As shown, its mass spectrum is shown as figure 2 shown.
[0069] IR(ν,cm-1):v(NH)br 3188,3073cm-1,vs(CH),vas(CH):2931,2855cm-1, δ(NH):1448cm-1,v(C-O): 1698,1615cm-1, v(C-C):1177cm-1, v(Pt-O):897cm-1, v(Pt-Cl):694cm-1, v(Pt-N):638cm-1.
[0070] 1H NMR(600MHz,DMSO):δ3.12-3.57(s,3H,CH3),2.65-2.87(s,1H,CH), 1.46-2.22(m,6H,CH2),0.97-1.38(m,4H ,CH2).
[0071] HR-MS (m / z): [C8H19Cl3N2O2Pt+H]+=476.0233 (10...
Embodiment 2
[0072] Embodiment 2: Preparation of compound (C-2) shown in formula III, namely:
[0073]
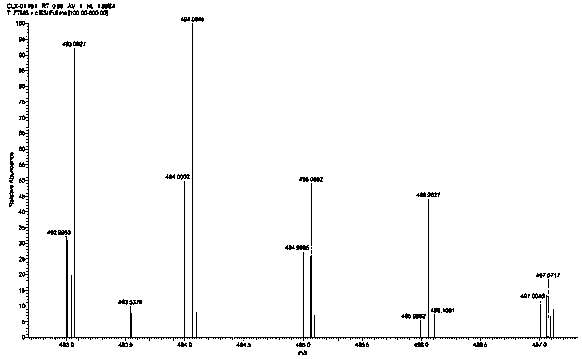
[0074] Dissolve 1 g (1.1 mol) of the Pt(II) compound prepared in Step 5 in 20 ml of acetic acid, weigh 0.34 g (1.1 mol) of N-chlorosuccinimide and add it to the reaction system, and stir evenly at room temperature overnight. After the reaction was completed, the solvent was evaporated and concentrated under reduced pressure, and freeze-dried to obtain 0.33 g of a Pt(IV) complex having a lipophilic group in the axial direction. Yield: 26.70%. Its hydrogen spectrum is as image 3 As shown, its mass spectrum is shown as Figure 4 shown.
[0075] IR(ν,cm-1):v(NH)br 3197,3076cm-1,vs(CH),vas(CH):2935,2857cm-1, δ(NH):1451cm-1,v(C-O): 1674,1613cm-1, v(C-C):1298cm-1, v(Pt-O):916cm-1, v(Pt-Cl):697cm-1, v(Pt-N):535cm-1.
[0076] 1H NMR(600MHz,DMSO):δ6.38-6.52(m,3H,NH3),2.69-2.79(s,1H,CH),2.00-2.10(m,2H,NH2),1.93-1.97(s,3H ,CH3),1.51-1.72(m,4H,CH2), 1.05-1.44(m,6H,CH2).
[0077] HR-MS (m / ...
Embodiment 3
[0078] Embodiment 3: Preparation of compound (C-3) shown in formula IV, namely:
[0079]
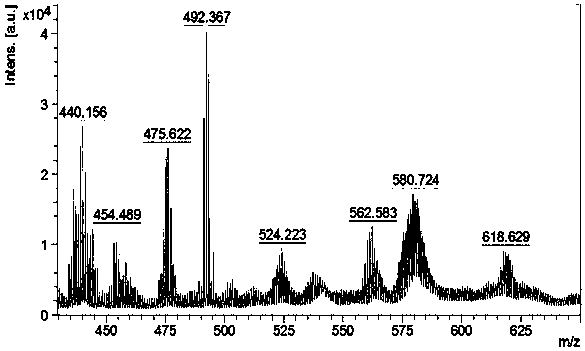
[0080] Dissolve 0.96g (1.1mol) of the Pt(II) compound prepared in step 4 in 20mL of acetic acid, weigh 0.28g (1.1mol) of N-chlorosuccinimide and add it to the reaction system, and stir evenly at room temperature overnight . After the reaction was completed, the solvent was evaporated and concentrated under reduced pressure, and freeze-dried to obtain 0.35 g of a Pt(IV) complex having a lipophilic group in the axial direction. Yield: 30.17%. Its hydrogen spectrum is as Figure 5 As shown, its mass spectrum is shown as Image 6 shown.
[0081]IR(ν,cm-1):v(NH)br 3164,3078cm-1,vs(CH),vas(CH):2939,2858cm-1, δ(NH):1454cm-1,v(C-O): 1680,1655cm-1, v(C-C):1208cm-1, v(Pt-O):901cm-1, v(Pt-Cl):697cm-1, v(Pt-N):573cm-1.
[0082] 1H NMR(600MHz,DMSO):δ6.08-6.39(m,4H),2.64-2.70(s,1H,CH), 2.44-2.46(m,3H,NH3),1.99-2.07(m,2H), 1.89-1.91(s,3H,CH3),1.75-1.82(m,2H,CH2),1.48-1.70(m,4H,CH2),1.19-1.33(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com