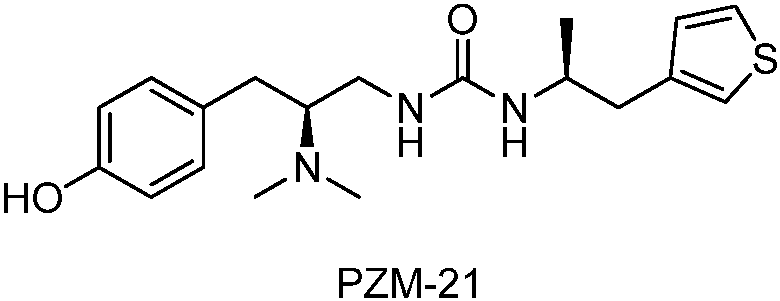
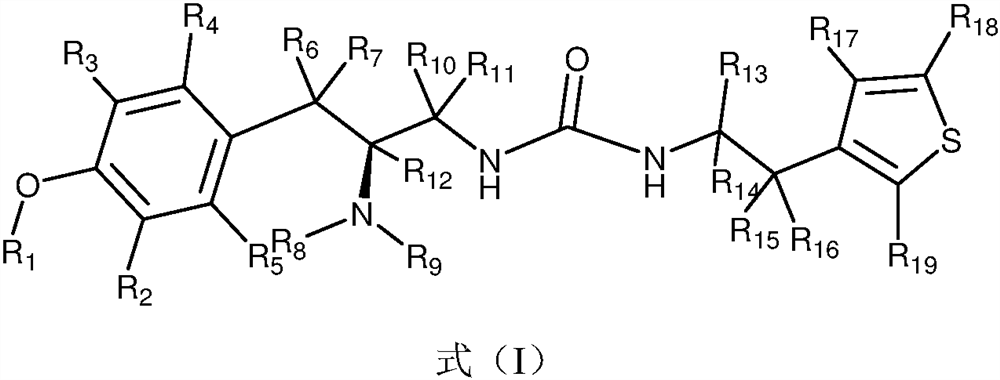
Novel deuterated urea compounds
A technology for compounds and compositions, applied in the field of medicine, which can solve the problems of variable, unpredictable results, and reduced metabolic clearance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
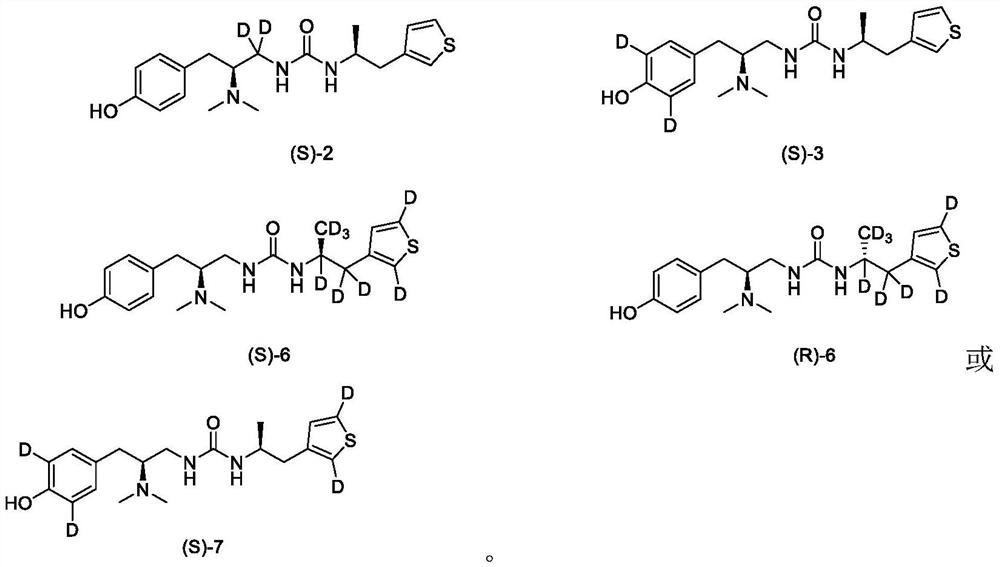
[0137] Example 1. Preparation of 1-((S)-2-(two (methyl-d 3 )amino)-3-(4-hydroxyphenyl)propyl)-3-((S)-1-(thiophen-3-yl)propane-2-yl)urea (compound (S)-1)
[0138]
[0139] step 1)
[0140] To the reaction flask was added (s)-2-amino-3-(4-hydroxyphenyl)propanamide (3.00 g, 16.65 mmol), acetonitrile (35 ml), and then 20% d 2 - formaldehyde heavy aqueous solution (27ml, 166.50mmol), stirring at room temperature; at the same time, add sodium borodeuteride (4.06g, 96.99mmol) and acetonitrile (5ml) to another reaction flask, add deuterium dropwise to the reaction at 0°C Acetic acid (20ml, 290.97mmol); the obtained d 1 - Sodium triacetoxy borodeuteride suspension was added in portions to the previous reaction mixture. After TLC monitors that the reaction is complete, add saturated aqueous sodium bicarbonate solution to the reaction to quench the reaction, then extract with isopropanol:ethyl acetate=1:3 (30ml*4) to obtain the organic phase, add anhydrous magnesium sulfate to dry,...
Embodiment 2
[0151] Example 2. Preparation of 1-((S)-2-(dimethylamino)-3-(4-hydroxyphenyl)propyl-1,1-d 2 )-3-((S)-1-(thiophen-3-yl)propan-2-yl)urea (compound (S)-2)
[0152]
[0153] step 1)
[0154] Add compound I-1 (1.00g, 4.80mmol) and anhydrous tetrahydrofuran (10ml) to the reaction flask, and stir at 0°C for 30 minutes under nitrogen protection; add 1.0M BD3-THF (28.90ml, 28.90mmol), heated to reflux overnight after the dropwise addition. After the completion of the reaction was monitored by TLC, methanol was slowly added at low temperature to quench the reaction. The reaction solution was spin-dried, and then methanol was added for rotary evaporation (10ml*3), then concentrated hydrochloric acid (1.0ml, 12mmol) was added, and ethanol was strip-evaporated (10ml*3). Acetone (10ml) was added into the reaction flask, and a solid precipitated out. Compound II-3 (0.674 g) was obtained by suction filtration and drying under reduced pressure at 45°C.
[0155] 1 H-NMR (500M, DMSO-d ...
Embodiment 3
[0161] Example 3. Preparation of 1-((S)-2-(dimethylamino)-3-(4-hydroxyphenyl-3,5-d 2 )Propyl)-3-((S)-1-(thiophen-3-yl)propan-2-yl)urea (compound (S)-3)
[0162]
[0163] step 1)
[0164] Add trifluoroacetic anhydride (1.5 g, 7.41 mmol) to a microwave reaction flask, and slowly add heavy water (15 ml) dropwise therein under an ice-water bath; add compound II-1 (0.8, 2.99 mmol) to the above reaction solution. Under microwave conditions, 160°C, 150Power, react for two hours. After the reaction, the heavy aqueous solution was removed by rotary evaporation, and the same amount of trifluoroacetic anhydride and heavy water as before were added, and exchanged again under the same reaction conditions. After the reaction was completed, the solvent was removed by rotary evaporation, acetone was added to make a slurry, and finally compound II-4 (0.654 g) was obtained by drying under reduced pressure.
[0165] 1 H-NMR (500M, DMSO-d 6 ): δ=11.10(br, 1H), 9.45(br, 1H), 8.51(br, 2H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com