Method for preparation of sugammadex sodium with polymer-loaded trivalent phosphine compound
A technology of sugammadex sodium and phosphine compounds, which is applied in the pharmaceutical field, can solve the problems of synthesis and purification of sugammadex sodium, which have not been seen, and achieve the effects of high environmental protection significance and economic value, easy purification, and improved economy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
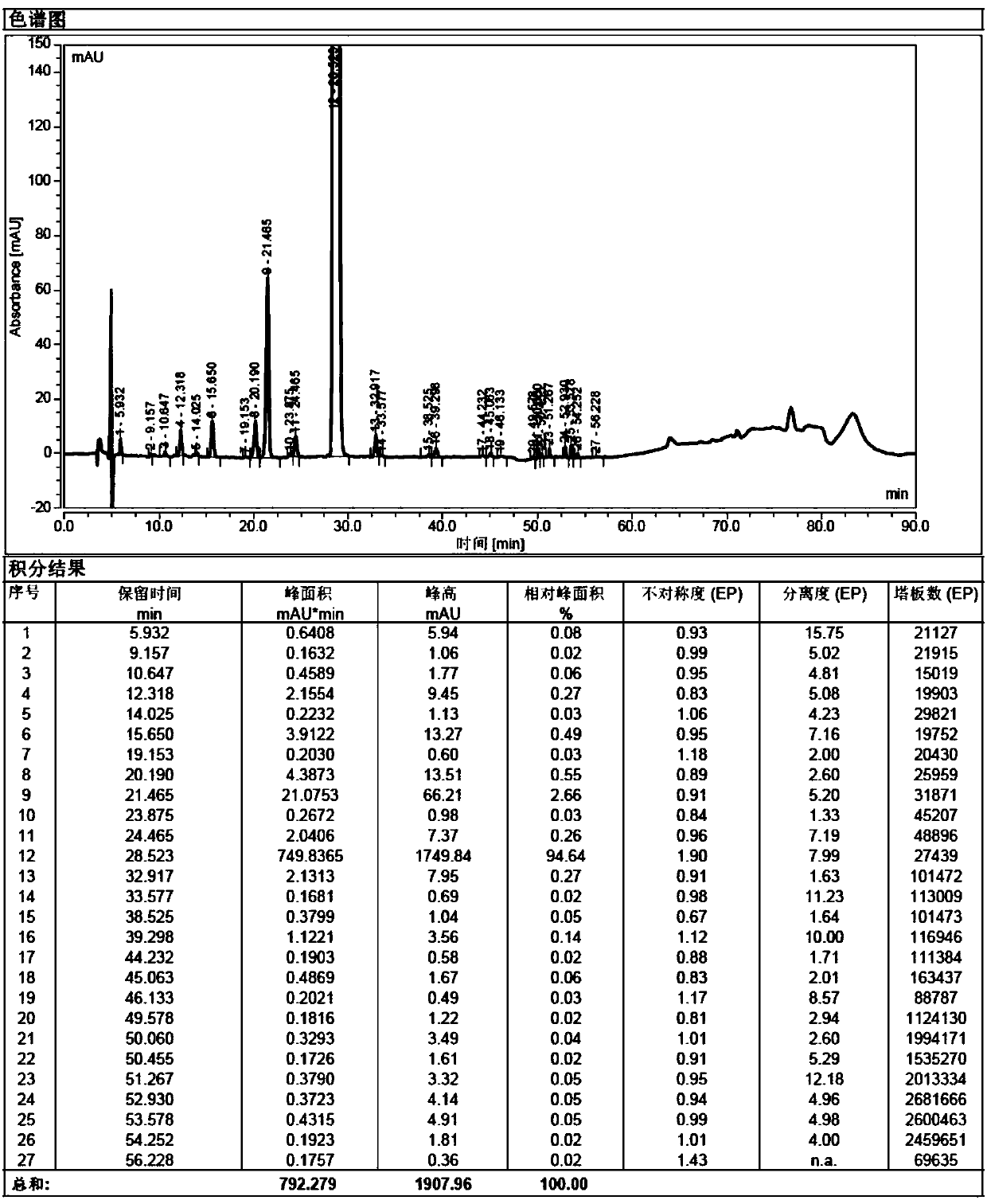
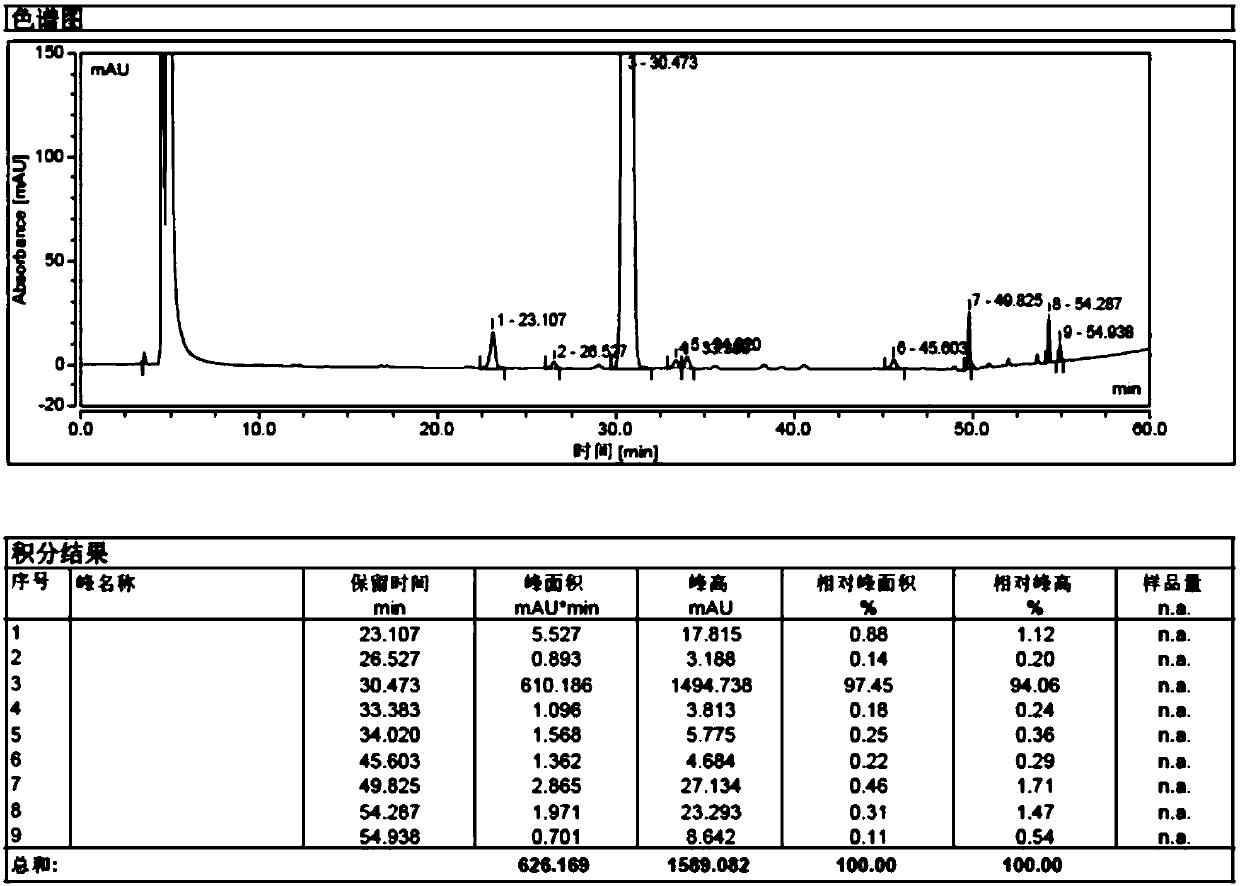
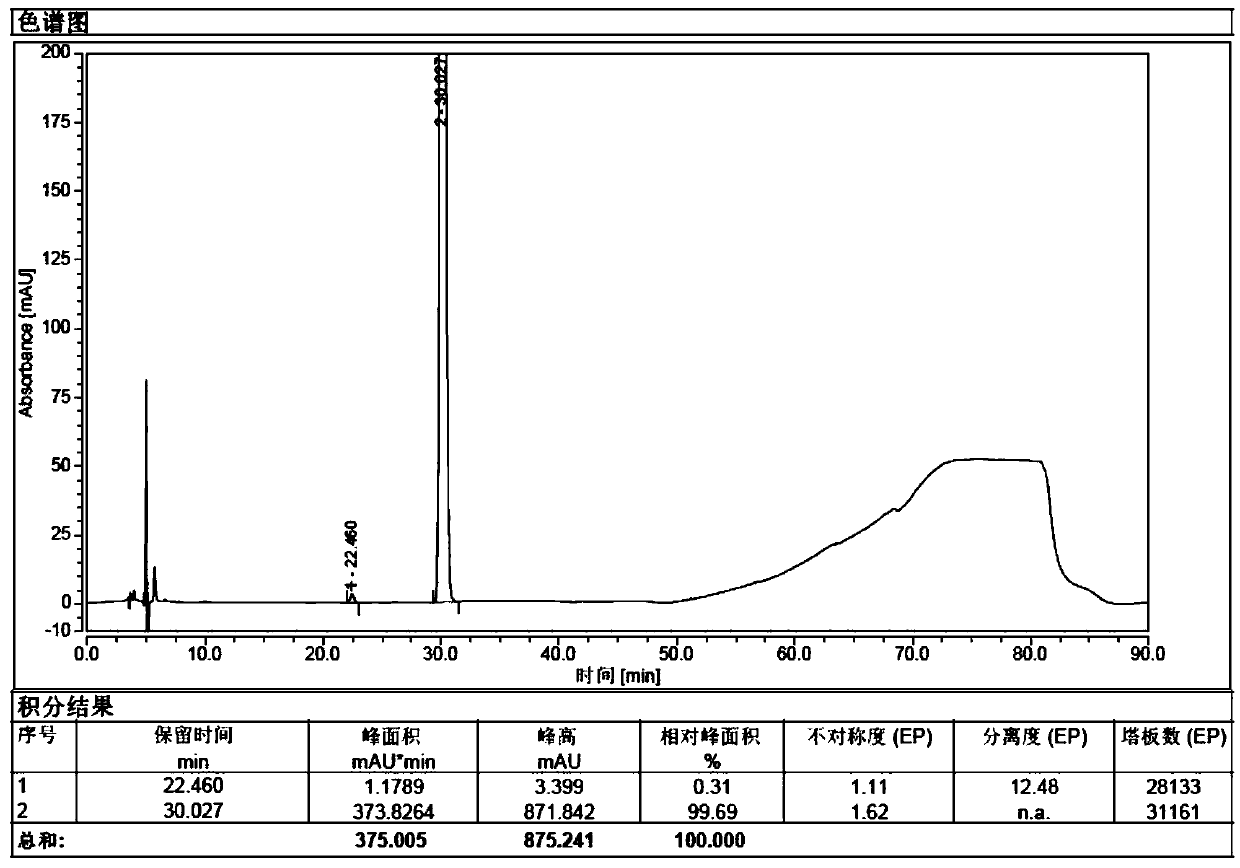
Image
Examples
Embodiment 1
[0056] 1. Synthesis of 6-deoxy-6-periodo-γ-cyclodextrin
[0057] Under stirring, add cross-linked polystyrene supported triphenylphosphine resin (60.2g), anhydrous DMF (160ml) and iodine (30.5g, 15.6 equivalents) into the flask, and the system exotherms. Dried γ-cyclodextrin (10 g, 7.7 mmol) was added, and the temperature was raised to 70° C. to react for 24 hours. After the reaction is over, cool the system, remove the crosslinked polystyrene-loaded triphenylphosphine resin by suction filtration, add methanol solution of sodium methylate (3.1g sodium is dissolved in 50ml methanol) in the filtrate, stir for 30 minutes, and add it to 800ml methanol medium, suction filtered, and the filter cake was dried. Add 500ml of water to the residue, filter with suction, wash the filter cake with water (3*100ml), and then wash with acetone (3*100ml), and dry it in vacuum at 70°C to obtain yellow 6-perdeoxy-6-periodo- Gamma-cyclodextrin solid (16.2 g). The recovered cross-linked polystyr...
Embodiment 2
[0063] 1. Synthesis of 6-deoxy-6-perbromo-γ-cyclodextrin
[0064] Under stirring, add cross-linked polystyrene supported triphenylphosphine resin (60.2g), anhydrous DMF (160ml) into the flask, and add bromine (19.2g, 15.6 equivalents) dropwise, and the system exotherms. Dried γ-cyclodextrin (10 g, 7.7 mmol) was added, and the temperature was raised to 70° C. to react for 24 hours. After the reaction is over, cool the system, remove the crosslinked polystyrene-loaded triphenylphosphine resin by suction filtration, add methanol solution of sodium methylate (3.1g sodium is dissolved in 50ml methanol) in the filtrate, stir for 30 minutes, and add it to 800ml methanol medium, suction filtered, and the filter cake was dried. Add 500ml of water to the residue, filter with suction, wash the filter cake with water (3*100ml), and then wash with acetone (3*100ml), and dry it in vacuum at 70°C to obtain yellow 6-perdeoxy-6-perbromo- Gamma-cyclodextrin solid (13.2 g). The recovered cros...
Embodiment 3
[0070] 1. Synthesis of 6-deoxy-6-perchloro-γ-cyclodextrin
[0071] Under stirring, add cross-linked polystyrene supported triphenylphosphine resin (60.2g), anhydrous DMF (160ml) and carbon tetrachloride (18.5g, 15.6 equivalents) into the flask, and the system exotherms. Dried γ-cyclodextrin (10 g, 7.7 mmol) was added, and the temperature was raised to 60° C. to react for 40 hours. After the reaction is over, cool the system, remove the crosslinked polystyrene-loaded triphenylphosphine resin by suction filtration, add methanol solution of sodium methylate (3.1g sodium is dissolved in 50ml methanol) in the filtrate, stir for 30 minutes, and add it to 800ml methanol medium, suction filtered, and the filter cake was dried. Add 500ml of water to the residue, filter with suction, wash the filter cake with water (3*100ml), and then wash with acetone (3*100ml), and dry it in vacuum at 70°C to obtain yellow 6-perdeoxy-6-perchloro- Gamma-cyclodextrin solid (16.2 g). The recovered cro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com