Application of colorimetric probe
A technology of colorimetric probes and probes, applied in the field of probes, can solve problems such as complex structures, poor water solubility, and poor specificity, and achieve the effects of good water solubility, high selectivity, and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The application of the colorimetric probe of the present embodiment, the molecular formula of the colorimetric probe is C 21 h 23 NO 4 S; the structural formula of the colorimetric probe is:
[0026]
[0027] The colorimetric probe is used to detect the total content of hypochlorous acid and hypochlorite in the aqueous solution, and the detection method is:
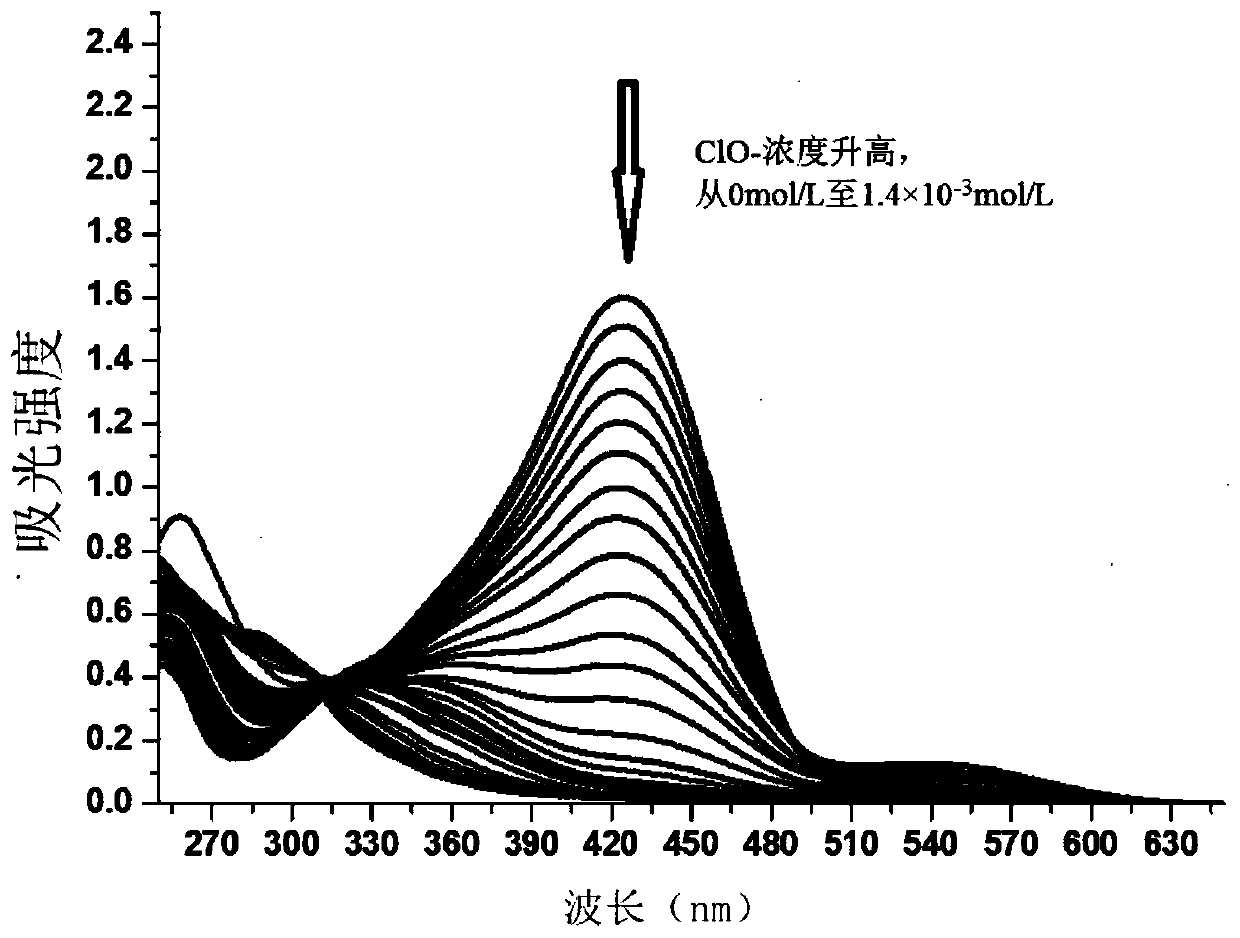
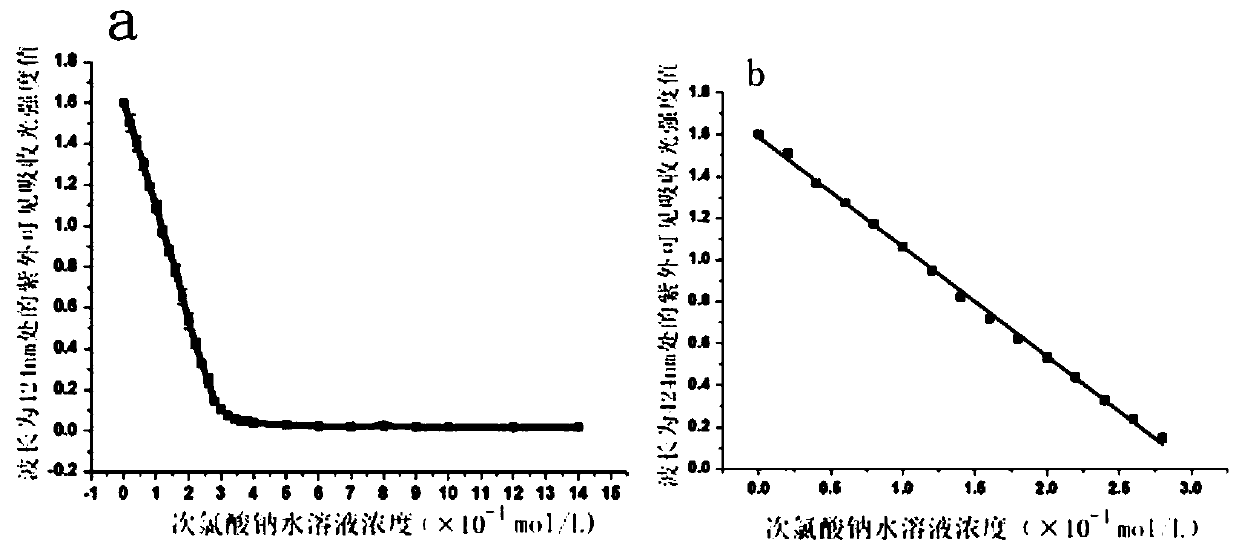
[0028] Step 1, dissolving the colorimetric probe in a phosphate buffer solution with a pH value of 5 at a concentration of 0.2mol / L to obtain a probe stock solution with a colorimetric probe of 0.5mmol / L, and then adding the probe stock solution to the probe stock solution Add 29 concentrations of 0mol / L to 1.4×10 -3 mol / L sodium hypochlorite aqueous solution, shake up and settle to 3mL with the phosphate buffer solution of 0.2mol / L pH value 5, obtain 29 standard solutions; The concentration of colorimetric probe in described 29 standard solutions is 0.1 mmol / L;
[0029] Step 2, measure the 29 standard solu...
Embodiment 2
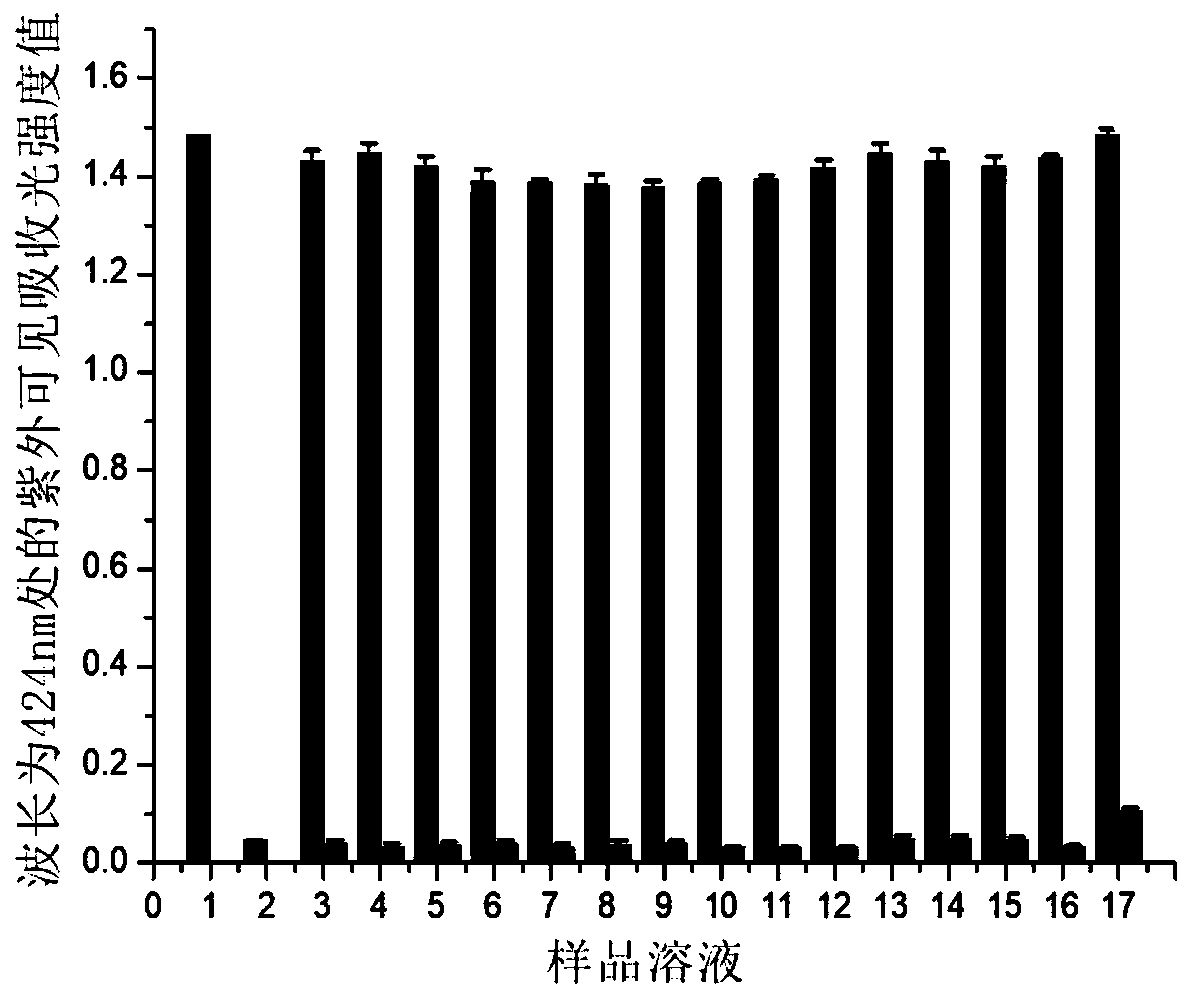
[0035] This embodiment is the selectivity of the colorimetric probe of embodiment 1 to metal cations:
[0036] Dissolve the colorimetric probe in a phosphate buffer solution with a pH value of 5 at a concentration of 0.2 mol / L to obtain a probe stock solution, and then add ClO with a concentration of 0.4 mmol / L to the probe stock solution. - Aqueous solution, metal ion Li + 、Co 2+ 、Cr 3+ 、K + 、Cd 2+ , Pb 2+ , Ca 2+ , Hg 2+ 、Ba 2+ 、Cu 2+ , Mg 2+ 、Ni 2+ , Zn 2+ 、Al 3+ and Fe 3+ Shake up and dilute to 3mL with 0.2mol / L phosphate buffer solution with a pH value of 5 to obtain 16 sample solutions, the concentration of the colorimetric probe in the 16 sample solutions is 0.1mmol / L ;
[0037] Contrast: dissolving the colorimetric probe in a phosphate buffer solution with a pH value of 5 at a concentration of 0.2mol / L to obtain a control probe solution with a colorimetric probe concentration of 0.1mmol / L; in the control probe solution Does not contain ClO - or metal i...
Embodiment 3
[0042] This embodiment is the selectivity of the colorimetric probe of embodiment 1 to anions:
[0043] Dissolve the colorimetric probe in a phosphate buffer solution with a pH value of 5 at a concentration of 0.2mol / L to obtain a probe stock solution, and then add ClO with a concentration of 0.4mmol / L to the probe stock solution. - Aqueous solution, other anions AcO - , ClO 4 - , SO 4 2- 、CN - 、Br - , NO 2 - , CO 3 2- , I - and F - Shake up the aqueous solution and dilute to 3mL with 0.2mol / L phosphate buffer solution of pH 5 to obtain 10 sample solutions, the concentration of the colorimetric probe in the 10 sample solutions is 0.1mmol / L;
[0044] Contrast: dissolving the colorimetric probe in a phosphate buffer solution with a pH value of 5 at a concentration of 0.2mol / L to obtain a control probe solution with a colorimetric probe concentration of 0.1mmol / L; in the control probe solution Does not contain ClO - or other anions;
[0045] Sample solutions of 9 o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com