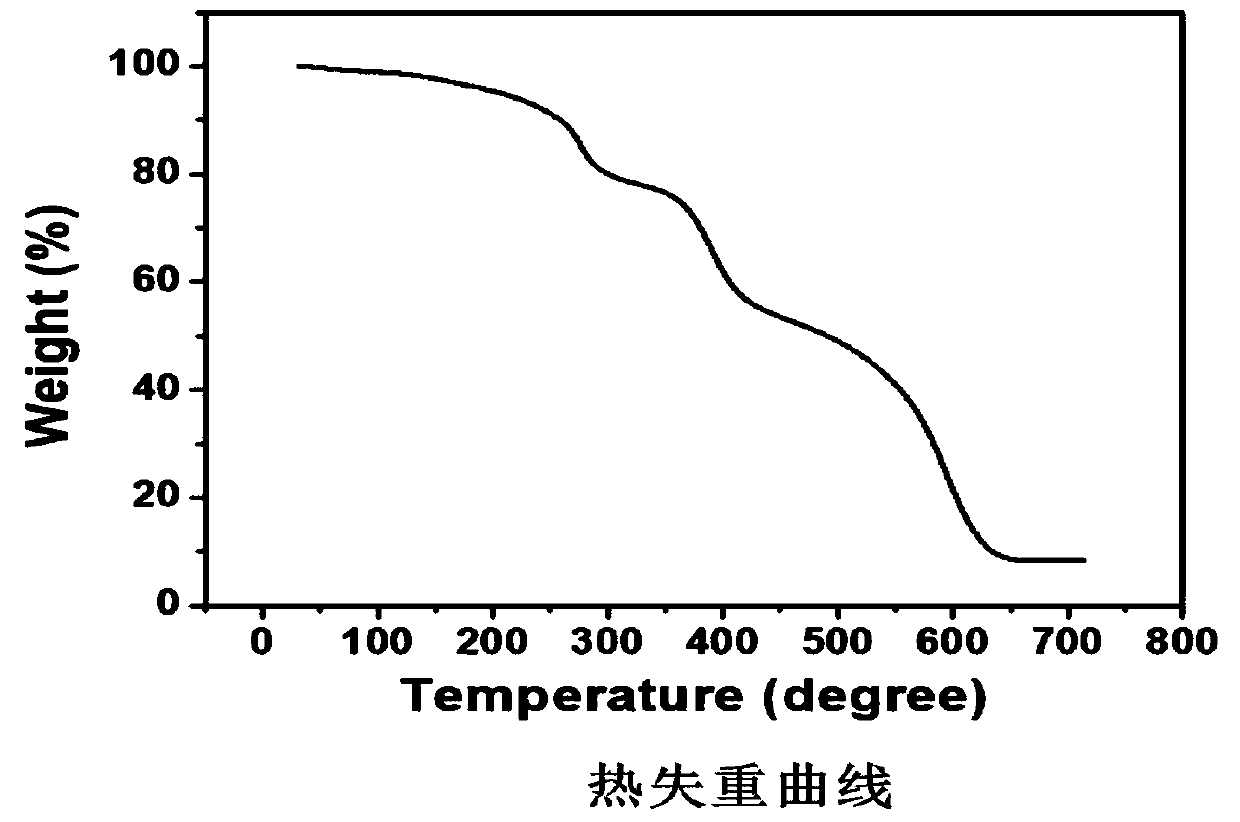
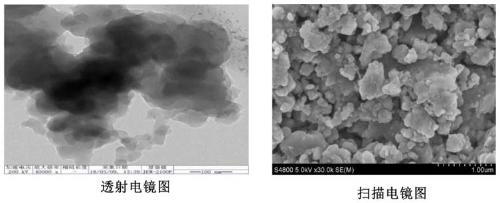
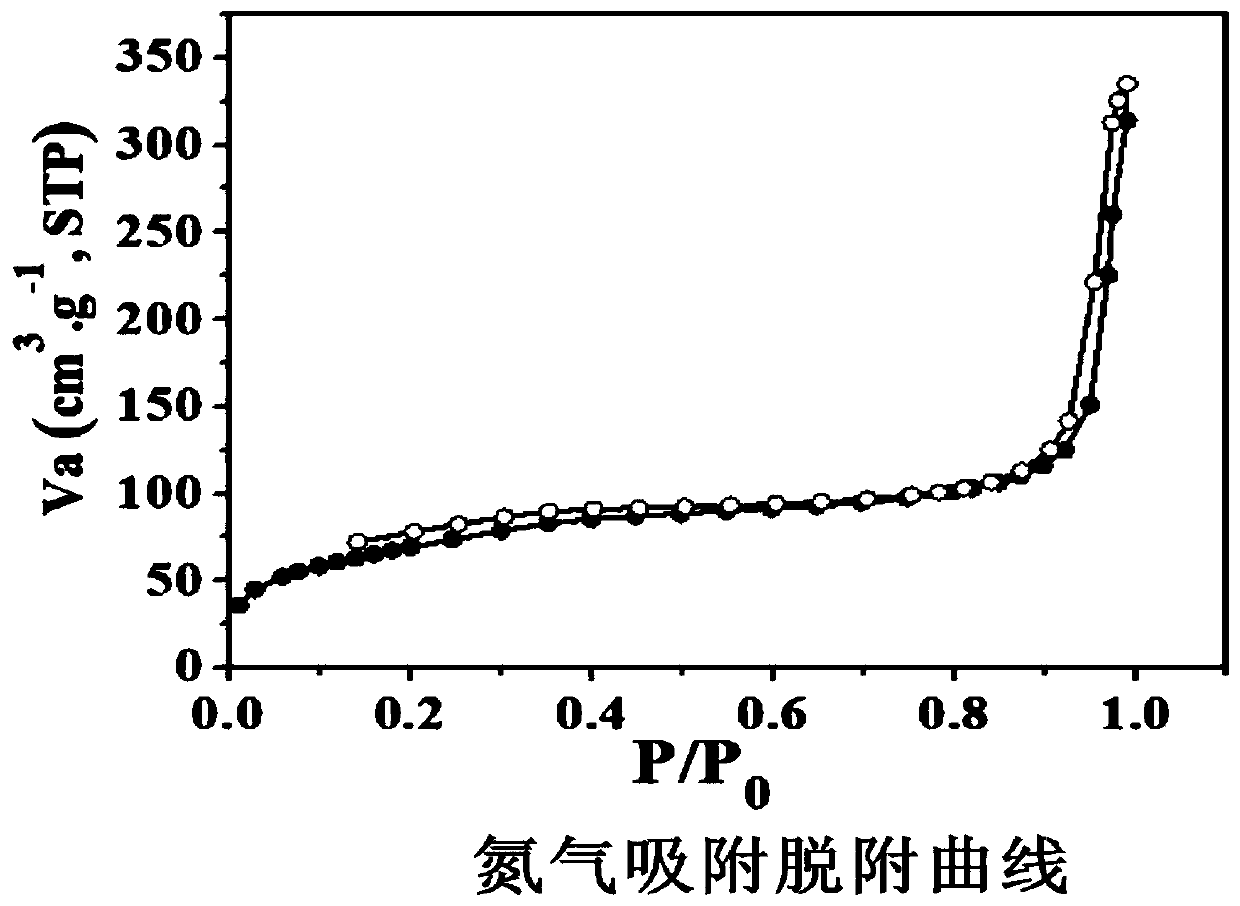
Preparation method and application of covalent organic framework material containing acylhydrazone bonds and disulfide bonds
A covalent organic framework and acylhydrazone bond technology, applied in the field of covalent organic framework polymer porous materials, can solve the problems that COFs cannot respond to acidic and reducing microenvironments at the same time, restricting the application of COFs, etc., achieve high load capacity, easy Control, operation simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of Covalent Organic Frameworks (COFs) Containing Acylhydrazone Bonds and Disulfide Bonds
[0028] Trimylene tricarbaldehyde (3.24mg, 0.20mmol) was placed in the reaction tube, followed by adding 5mL of mesitylene and dioxane (the volume ratio of mesitylene and dioxane was 3:1) in the reaction tube After the solution was mixed and fully dissolved, 4,4'-dithiodibenzohydrazide (100.2 mg, 0.3 mmol) was added to the tube. After fully dissolving, the catalyst aqueous acetic acid solution (0.6 mL, 5 mol / L) was added dropwise to the above solution. The sample was repeatedly cold-cut with liquid nitrogen-vacuum-thawed, then sealed under an anaerobic high vacuum, and placed in an oil bath at 120°C for 72 hours. After the reaction was completed, when the temperature of the system dropped to room temperature, precipitation appeared, and the solid product was separated by centrifugation, and washed repeatedly with tetrahydrofuran to remove unreacted monomers ...
Embodiment 2
[0034] Example 2: Preparation of covalent organic frameworks (COFs) containing acylhydrazone bonds and disulfide bonds
[0035] Trimylene tricarbaldehyde (3.24mg, 0.20mmol) was placed in the reaction tube, and 10mL of mesitylene and dioxane (the volume ratio of mesitylene and dioxane was 1:1) was added to the reaction tube After the solution was mixed and fully dissolved, 4,4'-dithiodibenzohydrazide (100.2 mg, 0.3 mmol) was added to the tube. After fully dissolving, the catalyst aqueous acetic acid solution (5 mL, 1 mol / L) was added dropwise to the above solution. The sample was repeatedly cold-cut with liquid nitrogen-vacuum-thawed, then sealed under anaerobic high vacuum, and placed in an oil bath at 150°C for 24 hours. After the reaction was completed, when the temperature of the system dropped to room temperature, precipitation appeared, and the solid product was separated by centrifugation, and washed repeatedly with tetrahydrofuran to remove unreacted monomers and solve...
Embodiment 3
[0036] Example 3: Preparation of Covalent Organic Frameworks (COFs) Containing Acylhydrazone Bonds and Disulfide Bonds
[0037] Trimylene tricarbaldehyde (3.24mg, 0.20mmol) was placed in the reaction tube, and 10mL of mesitylene and dioxane (the volume ratio of mesitylene and dioxane was 5:1) was added to the reaction tube After the solution was mixed and fully dissolved, 4,4'-dithiodibenzohydrazide (100.2 mg, 0.3 mmol) was added to the tube. After fully dissolving, the catalyst aqueous acetic acid solution (3 mL, 10 mol / L) was added dropwise to the above solution. The sample was repeatedly cold-cut with liquid nitrogen-vacuum-thawed, then sealed under an anaerobic high vacuum, and placed in an oil bath at 100°C for 48 hours. After the reaction was completed, when the temperature of the system dropped to room temperature, precipitation appeared, and the solid product was separated by centrifugation, and washed repeatedly with tetrahydrofuran to remove unreacted monomers and s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com