Application of NADH and/or NADPH in antiallergic drugs and/or antiallergic health products
A technology of anti-allergic and health care products, which is applied in the direction of drug combination, allergic diseases, and pharmaceutical formulas. It can solve problems such as adverse reactions, allergies requiring repeated medication, and easy recurrence, and achieve the effect of alleviating allergic symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] In this example, C3H / HeJ peanut-allergic mice were prepared by the following method: 6-week-old female C3H / HeJ mice were purchased from Shanghai Experimental Animal Center. The test animals are reared under specific non-pathogenic conditions in a room with an average temperature of 21° C. to 23° C. and a relative humidity of 40% to 70%, with a 12-hour light / dark cycle.
[0037] The mice in the sensitization group were gavaged with peanut homogenate (single 80mg / each) every day, and on the 21st day after the implementation of the sensitization measures, the mice were given intraperitoneal injection (i.p) injection of 30mg CPE (peanut extract) / hour Rats, and young rats that had never been exposed to peanuts served as negative controls. Then, by skin testing and anaphylaxis (by measuring vascular leak, monitoring clinical symptoms, rectal temperature, respiratory rate, and measuring serum mast cell protease-1 (mmcp-1) - a specific marker of mast cell degranulation) The assa...
Embodiment 2
[0057] C3H / HeJ peanut-allergic mice in the test example of the present invention were prepared by the following method: 6-week-old female C3H / HeJ mice were purchased from Shanghai Experimental Animal Center. The test animals were reared under specific non-pathogenic conditions in a room with an average temperature of 21°C-23°C and a relative humidity of 40%-70%, with a 12-hour light / dark cycle.
[0058] The mice in the sensitization group were gavaged with peanut homogenate (single 80mg / each) every day, and on the 21st day after the implementation of the sensitization measures, the mice were given intraperitoneal injection (i.p) injection of 30mg CPE (peanut extract) / hour Rats, and young rats that had never been exposed to peanuts served as negative controls. This is followed by skin testing and detection of anaphylaxis (by measuring vascular leak, monitoring clinical symptoms, rectal temperature, respiratory rate, and measuring serum mast cell protease-1 (mmcp-1) - a specific...
Embodiment 3
[0078] 3.1 Experimental materials and experimental reagents:
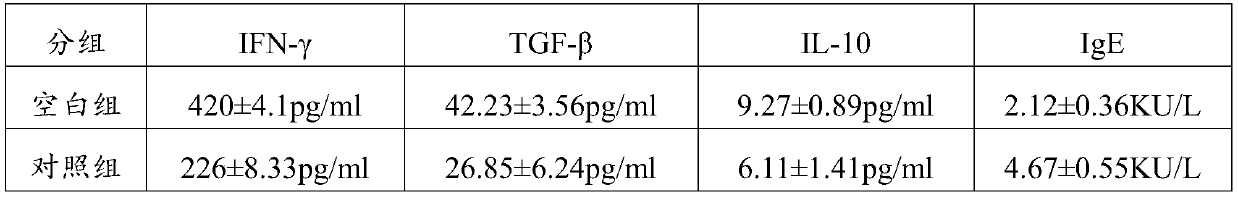
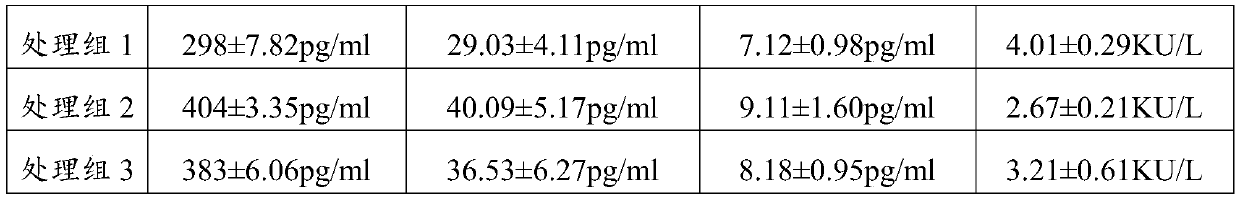
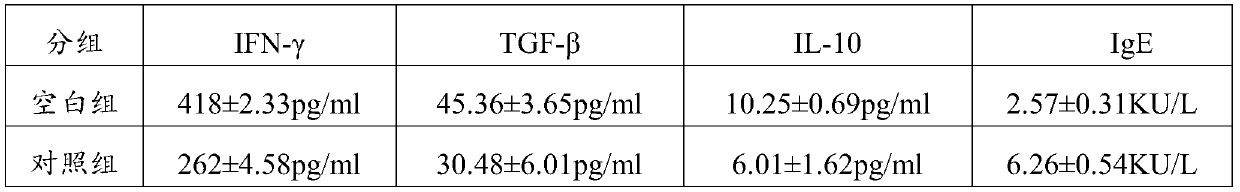
[0079] The peripheral blood of healthy children of the same age and the peripheral blood (PBMC) of children with food allergy were obtained from Shenzhen Children's Hospital. IL10, TGF-β, IFN-γ and IgE antibodies (purchased from Sigma-aldrich), NADH (self-prepared). 3.2 Experimental method
[0080] The peripheral blood (PBMC) of healthy children and food-allergic children were randomly collected, and the PBMC were suspended in a medium (RPMI-1640; Mediatech) with 10% autologous plasma, and incubated at 37°C, 5% CO 2 The culture groups in the cell culture incubator are as follows:
[0081] ①. Healthy group: PBMC (10^7 / ml) of healthy children were suspended in culture medium (adding physiological saline 0.2 mg / ml to the medium;
[0082] ②. Blank group: PBMC (10^7 / ml) from children with food allergy were suspended in culture medium (0.2 mg / ml of normal saline was added to the medium);
[0083] ③. Treatment group 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com