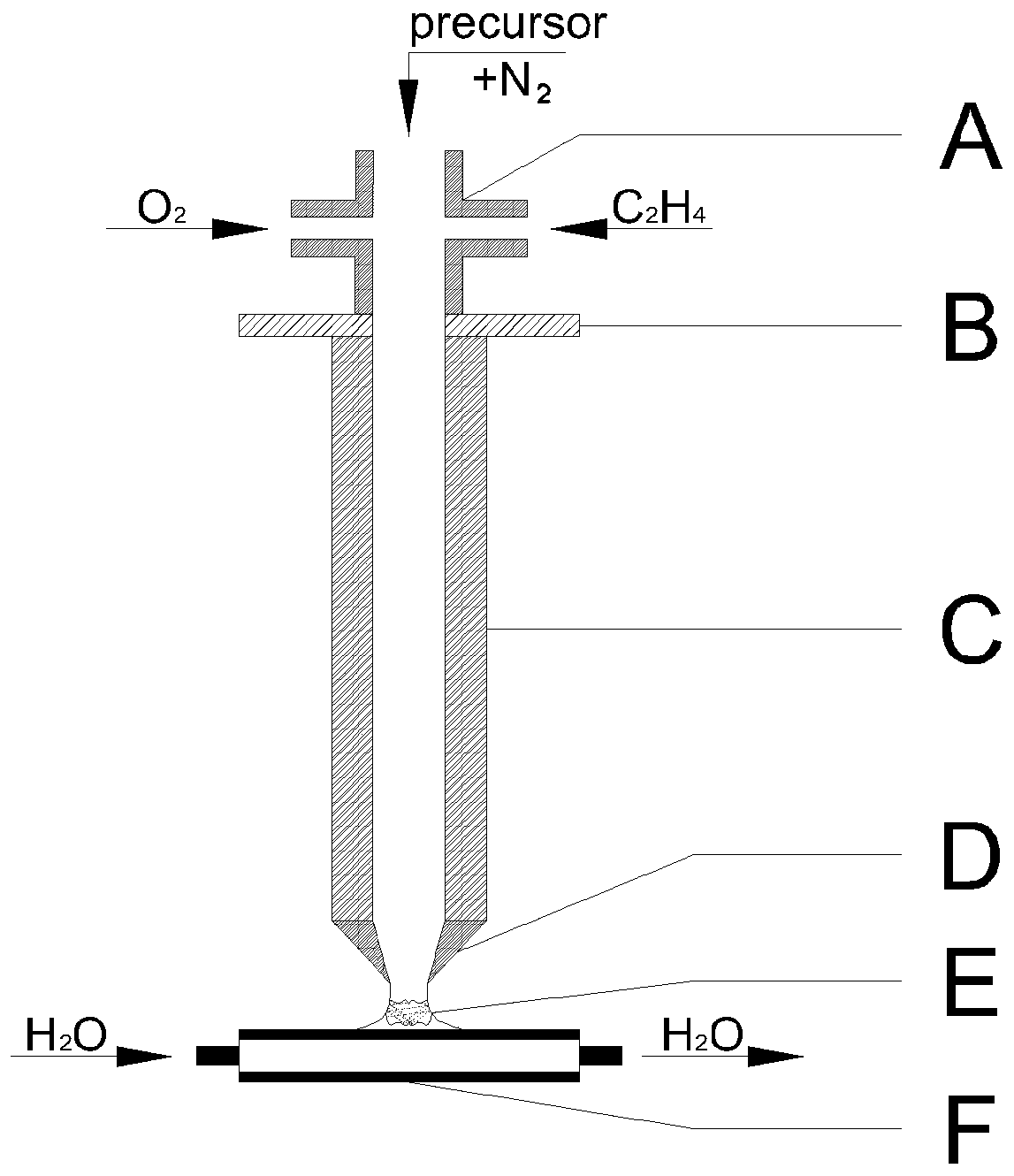
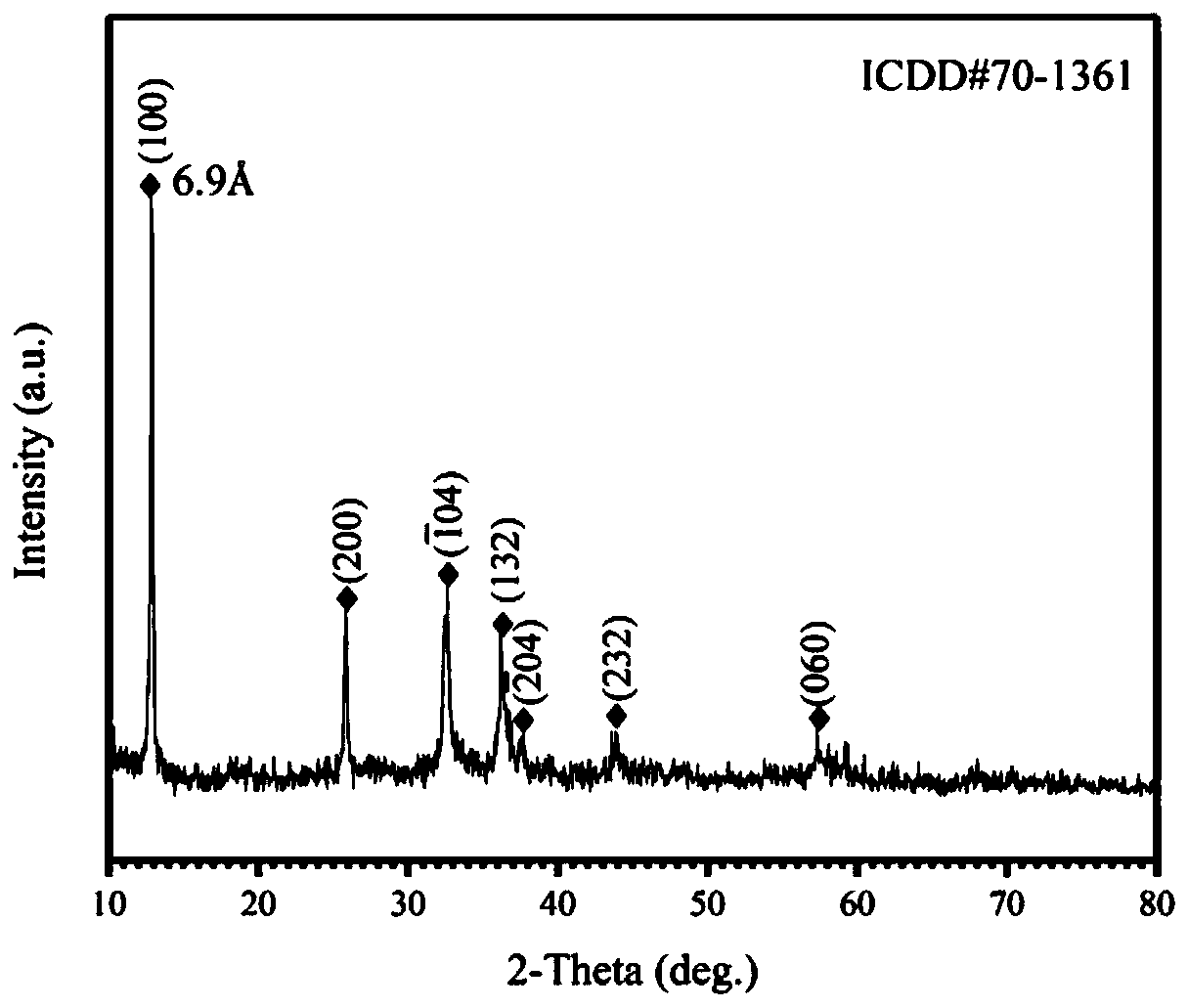
Ultra-thin zinc hydroxy nitrate nanosheet with single crystal Zn3(OH)4(NO3)2 structure and preparation method of nanosheet
A technology of hydroxyzinc nitrate and nanosheets, which is applied in the field of nanomaterials and can solve the problems of amorphous, polycrystalline and heterozygous
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] 50ml, 0.3M Zn(NO 3 ) 2 ·6H 2 After the O aqueous solution was prepared and mixed, in order to fully dissolve, the precursor solution was stirred with a magnetic stirrer at 30 °C for 30 min, and then loaded into a micro-injector. Before the experiment started, the air in the reaction device was exhausted with a large flow of nitrogen, and the water cooling system was turned on at the same time to carry out the water cooling process of circulating water, and the surface temperature of the solid phase receiving plate was controlled to maintain a stable value of about 40 °C, and then the ethylene concentration was adjusted. The gas flow rate is 0.6SLPM, the gas flow rate of oxygen is 5.4SLPM, and the gas flow rate of nitrogen is 9.TSLPM. After 10s, it is ignited at a distance of 15mm from the nozzle. In the presence of unstable phenomena such as diffusion and floating, turn on the micro-injector again, inject samples at a speed of 0.003SLPM, and bring the reactants into t...
Embodiment 2
[0065] Only the premix gas flow is changed. 50ml, 0.3M Zn(NO 3 ) 2 ·6H 2 After the O aqueous solution was prepared and mixed, in order to fully dissolve, the precursor solution was stirred with a magnetic stirrer at 30 °C for 30 min, and then loaded into a micro-injector. Before the experiment started, the air in the reaction device was exhausted with a large flow of nitrogen, and the water cooling system was turned on at the same time to carry out the water cooling process of circulating water, and the surface temperature of the solid phase receiving plate was controlled to maintain a stable value of about 40 °C, and then the ethylene concentration was adjusted. The gas flow rate is 0.4SLPM or 0.75SLPM, the gas flow rate of oxygen is 2.7SLPM or 5.8SLPM, and the gas flow rate of nitrogen gas is 8SLPM or 10.2SLPM. After 10s, it is ignited at a distance of 15mm from the nozzle. After fine-tuning, a flame zone is formed, and the flame is stable within 10 minutes Existence, no ...
Embodiment 3
[0068] Change the distance between the receiving plate and the reactor outlet and the temperature on the surface of the solid phase receiving plate. 50ml, 0.3M Zn(NO 3 ) 2 ·6H 2 After the O aqueous solution was prepared and mixed, in order to fully dissolve, the precursor solution was stirred with a magnetic stirrer at 40 °C for 30 min, and then loaded into a micro-injector. Before the start of the experiment, the air in the reaction device was exhausted with a large flow of nitrogen, and the water cooling system was turned on at the same time to carry out the water cooling process of circulating water, and the surface temperature of the solid phase receiving plate was controlled to maintain a stable value of about 70°C, and then the ethylene concentration was adjusted. The gas flow rate is 0.6SLPM, the gas flow rate of oxygen gas is 5.4SLPM, and the gas flow rate of nitrogen gas is 9.TSLPM. Adjust the distance between the receiving plate and the reactor outlet to 5mm or 30m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com