Solid acid catalyst for preparing acetal/ketal, and preparation method and application thereof
A solid acid catalyst and solid acid technology, applied in the direction of physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, chemical instruments and methods, etc., can solve problems such as industrial wastes and complex processes, and achieve high Selective, low dosage effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
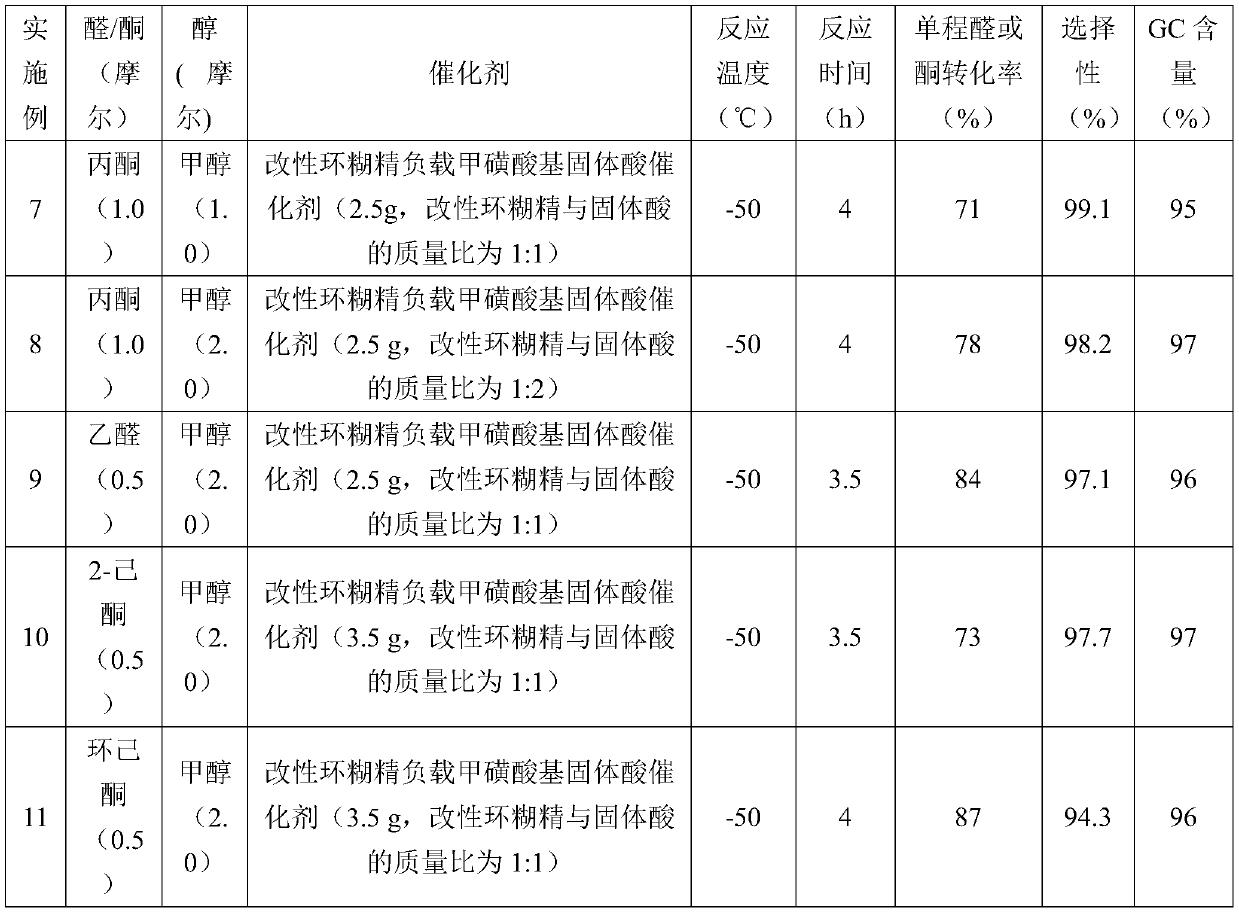
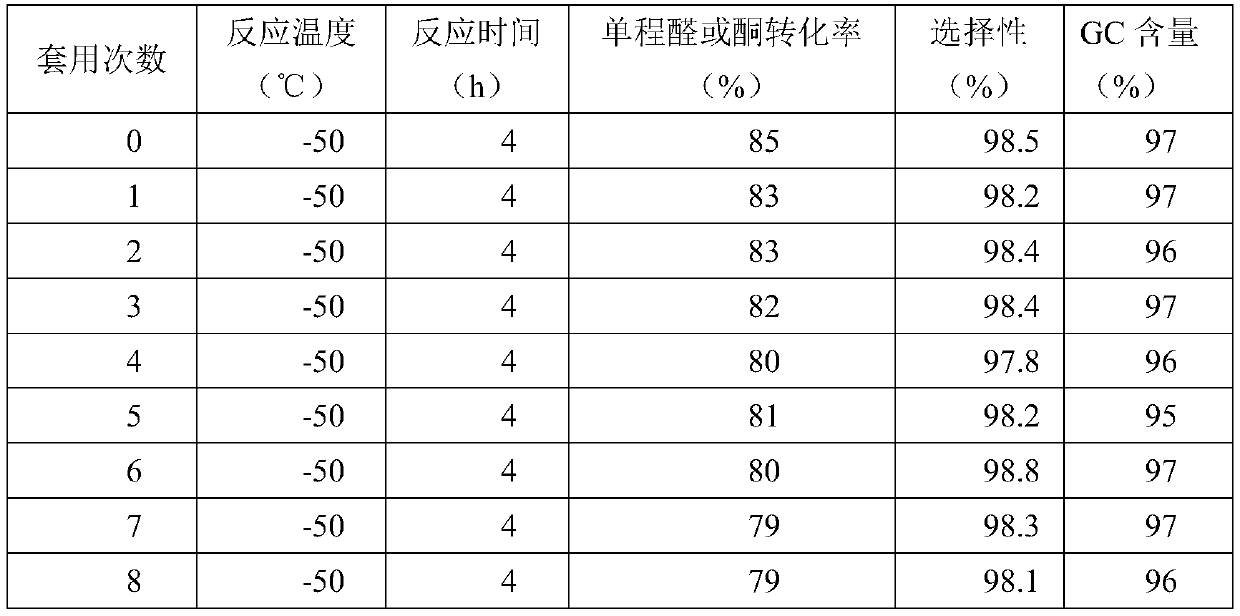
[0045] Add 2.5 g of a modified cyclodextrin-supported mesylate-based solid acid catalyst into the reaction kettle (in the catalyst, the mass ratio of the modified cyclodextrin to the solid acid is 1:1), and pre-cool to -50°C. Mix 0.5 moles of acetone and 2.0 moles of methanol in a mixing tank, then cool down to -50°C, add them into the reaction kettle, and stir and react at -50°C for 4 hours. After the reaction was completed, the reaction solution was obtained by filtration and separation. The reaction liquid is subjected to rectification, and methanol and acetone are firstly separated to obtain methanol and acetone, which are returned to the raw material mixing tank and used mechanically; and then the product 2,2-dimethoxypropane is obtained by rectification and dehydration. The one-way acetone conversion rate is 85%, the product selectivity is 98.5%, and the GC content is 97%.
Embodiment 2
[0047] Add 3.0 g of a modified cyclodextrin-supported mesylate-based solid acid catalyst into the reaction kettle (in the catalyst, the mass ratio of the modified cyclodextrin to the solid acid is 1:1.5), and pre-cool to -50°C. Mix 0.5 moles of acetone and 2.0 moles of ethanol in a mixing tank, then cool down to -50°C, add them into the reaction kettle, and stir and react at -50°C for 4 hours. After the reaction was completed, the reaction solution was obtained by filtration and separation. The reaction liquid is subjected to rectification, and ethanol and acetone are firstly separated to obtain ethanol and acetone, which are returned to the raw material mixing tank and used mechanically; and then the product 2,2-diethoxypropane is obtained by rectification and dehydration. The one-way acetone conversion rate is 87%, the product selectivity is 99.0%, and the GC content is 97%.
Embodiment 3
[0049] Add 2.5 g of a modified cyclodextrin-supported mesylate-based solid acid catalyst into the reaction kettle (in the catalyst, the mass ratio of the modified cyclodextrin to the solid acid is 1:0.5), and pre-cool to -30°C. Mix 0.5 moles of acetone and 1.0 moles of methanol in a mixing tank, then cool down to -30°C, add them into the reaction kettle, and stir and react at -30°C for 2 hours. After the reaction was completed, the reaction solution was obtained by filtration and separation. The reaction liquid is subjected to rectification, and methanol and acetone are firstly separated to obtain methanol and acetone, which are returned to the raw material mixing tank and used mechanically; and then the product 2,2-dimethoxypropane is obtained by rectification and dehydration. The one-way acetone conversion rate is 63%, the product selectivity is 97.6%, and the GC content is 97%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com