Fluorescent probe for detecting carbon monoxide and application thereof
A fluorescent probe, carbon monoxide technology, applied in the field of analytical chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Synthesis of Fluorescent Probes
[0034] (1) Synthesis of N-(2-aminoethyl)-4-methylphenyl-1-sulfonamide:
[0035]
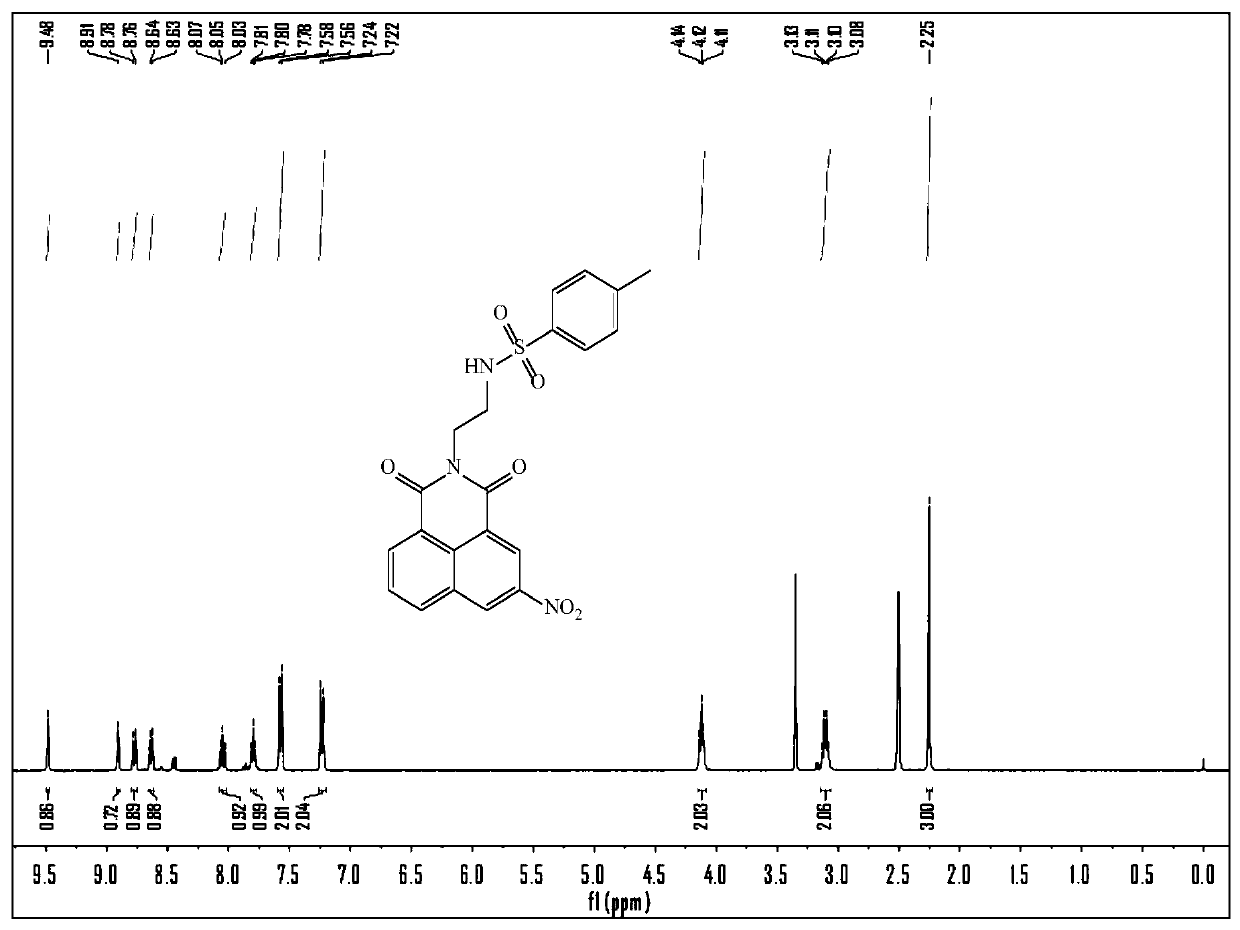
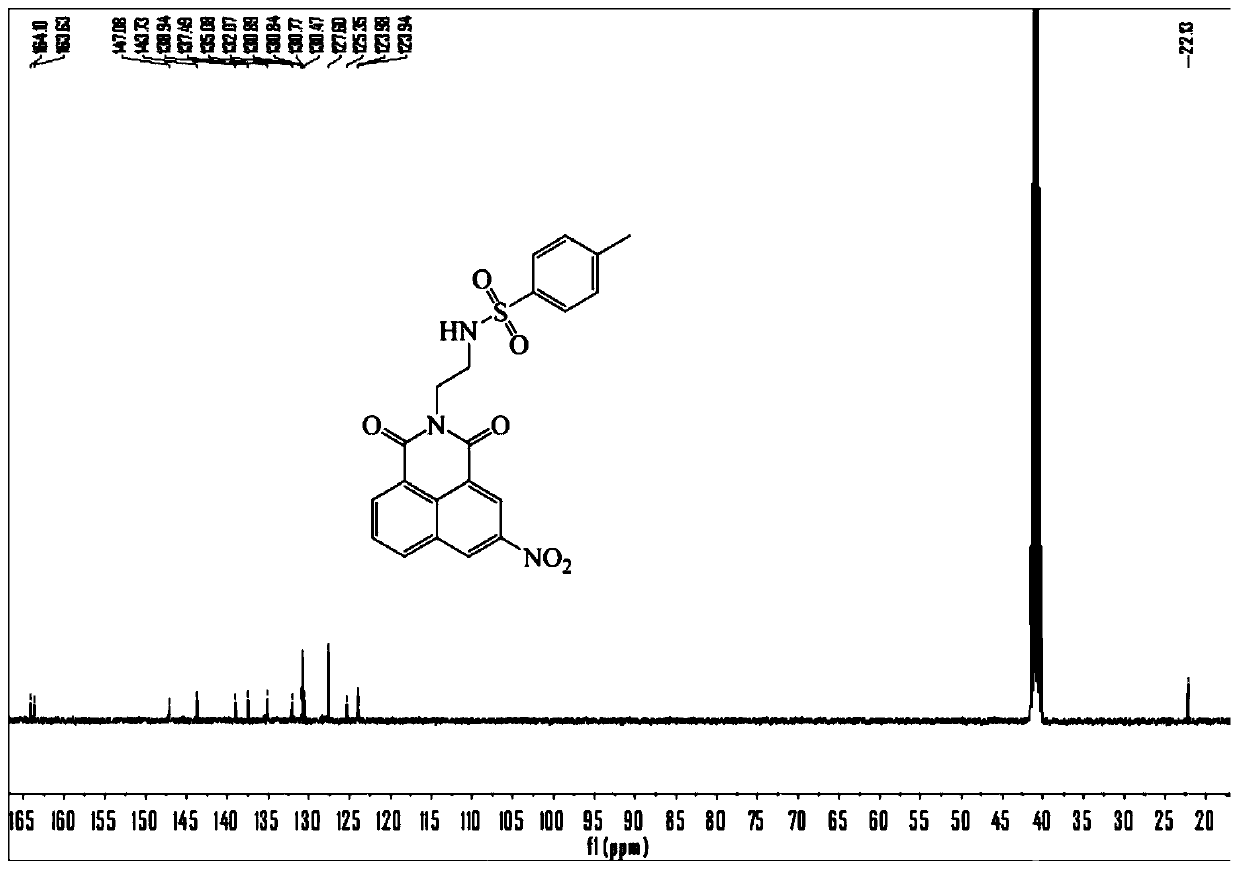
[0036] Dissolve ethylenediamine (10 mmol) in 10 mL of dichloromethane, and add 30 mmol of triethylamine and 10 mL of dichloromethane dissolved in 4-toluenesulfonyl chloride (7.5 mmol) at 0°C, at room temperature Stir for 12 hours, then add 50 mL of dichloromethane, wash with 15% potassium bicarbonate solution and ultrapure water for 2-3 times, separate the organic phase, evaporate the organic solvent under reduced pressure, separate by column chromatography, rinse The solvent is petroleum ether / dichloromethane (V / V=50:1-10:1), and the compound N-(2-aminoethyl)-4-methylphenyl-1-sulfonamide (1) is obtained, and the product Rate: 83%. That 1 H NMR (400 MHz, CDCl 3 ) δ 7.75 (d, J = 8.0 Hz, 2H), 7.30 (d, J = 8.0 Hz, 2H), 2.96 (t, J = 5.6 Hz, 2H), 2.79 (t, J = 5.6 Hz, 2H), 2.42 (s, 3H); 13 C NMR (100 MHz, CDCl 3 ) δ 143.4, 136.9, 129.8, 1...
Embodiment 2
[0040] Example 2 Responses of fluorescent probes to different concentrations of CO
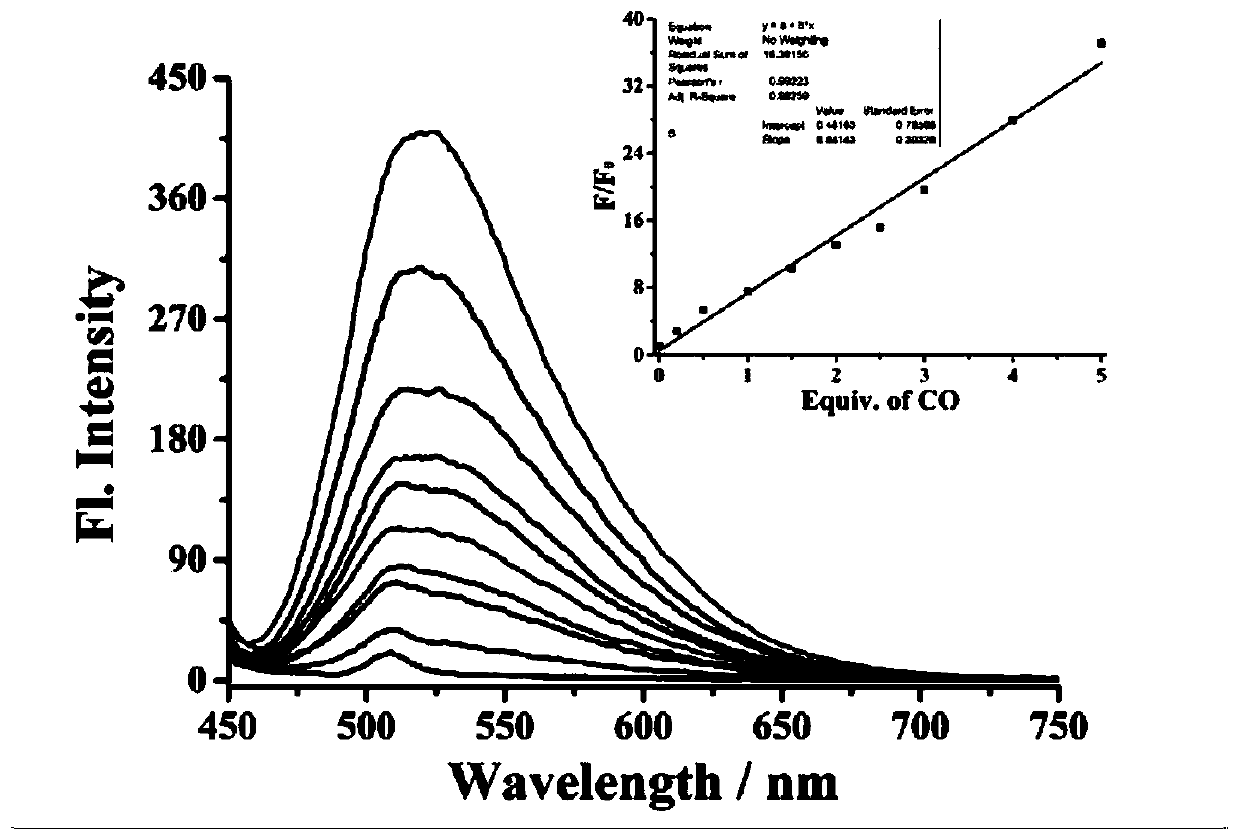
[0041] Prepare 5 mL of an aqueous solution with a concentration of 1 mM CO and the fluorescent probe mother solution prepared in Example 1 with a concentration of 1 mM as spares. Prepare the probe at a concentration of 10 µM, interact with different concentrations of CO (0-50 µM), and perform fluorescence detection (λ ex = 430 nm, λ em = 525 nm), calculate the fluorescence intensity in each system, and establish the standard curve of fluorescence intensity and CO concentration. like image 3 As shown, within the test concentration range, with the increase of CO concentration, the fluorescence intensity of the reaction system gradually increases linearly.
Embodiment 3
[0042] Example 3 Response kinetics of fluorescent probes to different concentrations of CO
[0043] Prepare 5 mL of an aqueous solution with a concentration of 1 mM CO and a mother solution of a fluorescent probe for detecting CO of the present invention with a concentration of 1 mM as backup. The solutions of probe and CO were prepared, the concentrations were: probe 10 μM; CO concentration: 0, 25, 50 μM. Perform fluorescence detection (λ ex = 430 nm, λ em = 525 nm), tested every 1 min for 60 min, calculated the fluorescence intensity in each system over time, and established a standard curve of fluorescence intensity and action time. like Figure 4 As shown, the fluorescence intensity of the reaction system reached saturation after about 50 min of reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com