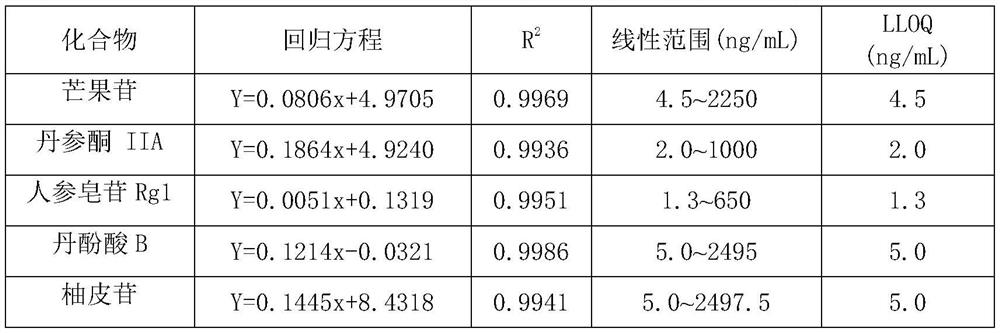
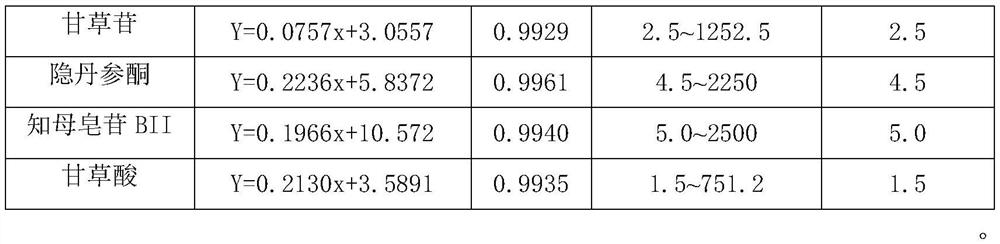
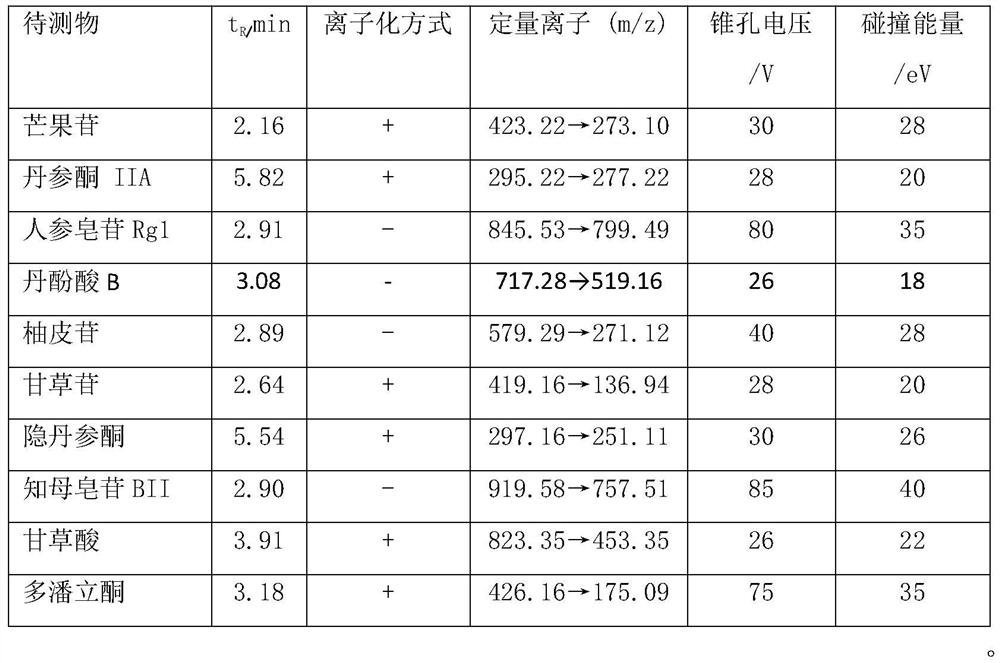
A method for simultaneous quantitative detection of main components of Shuangshen Pingfei Granules in plasma
A quantitative detection and plasma technology, which is applied in the field of medicine and Chinese patent medicine in vivo metabolism research, can solve the problems that have not been reported, and achieve good sensitivity and accurate and reliable results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1: Establishment of a method for determining the content of main components in rat plasma after oral administration of Shuangshen Pingfei Granules
[0077] Include the following steps:
[0078]Administration and collection of plasma samples
[0079] The rats were fed adaptively for one week, fasted for 12 hours before the experiment, had free access to water, and ate uniformly after 8 hours of administration. The next day, the experimental animals were randomly divided into 2 groups, 10 in each group, administered with Shuangshen Pingfei Granules by intragastric administration respectively at doses of 5 g / kg and 15 g / kg, respectively at 5, 15, 30 min and 1, 1.5, 2, 4, 8, 12, and 24 hours, 0.3 mL of blood was collected from the venous plexus behind the eyes. The collected blood samples were collected in EP tubes filled with sodium heparin, incubated in a 37°C water bath for 30 minutes, centrifuged at 5000rmp for 10 minutes, separated from the plasma, and stored...
Embodiment 2
[0121] Pharmacokinetics of main components in rat plasma after oral administration of Shuangshen Pingfei Granules
[0122] 1. Administration and collection of plasma samples
[0123] The rats were fed adaptively for one week, fasted for 12 hours before the experiment, had free access to water, and ate uniformly after 8 hours of administration. The next day, the experimental animals were randomly divided into 2 groups, 10 in each group, and the above-mentioned Shuangshen Pingfei Granules were administered by intragastric administration at doses of 5g / kg and 15g / kg respectively. After administration, 5, 15, 30min and 1 , 1.5, 2, 4, 8, 12, and 24 hours, 0.3 mL of blood was collected from the venous plexus behind the eyes. The collected blood samples were collected in EP tubes filled with sodium heparin, incubated in a 37°C water bath for 30 minutes, centrifuged at 5000rmp for 10 minutes, separated from the plasma, and stored in a -80°C refrigerator for testing.
[0124] 3.2 Pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com