Application of benzo-six-membered ring derivatives as DPP-4 long-acting inhibitors
A kind of DPP-4, inhibitor technology, applied in the application field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1. Synthesis of Compounds
[0050] The compound of the present invention was synthesized with reference to the applicant's prior application CN 105566276A.
Embodiment 2
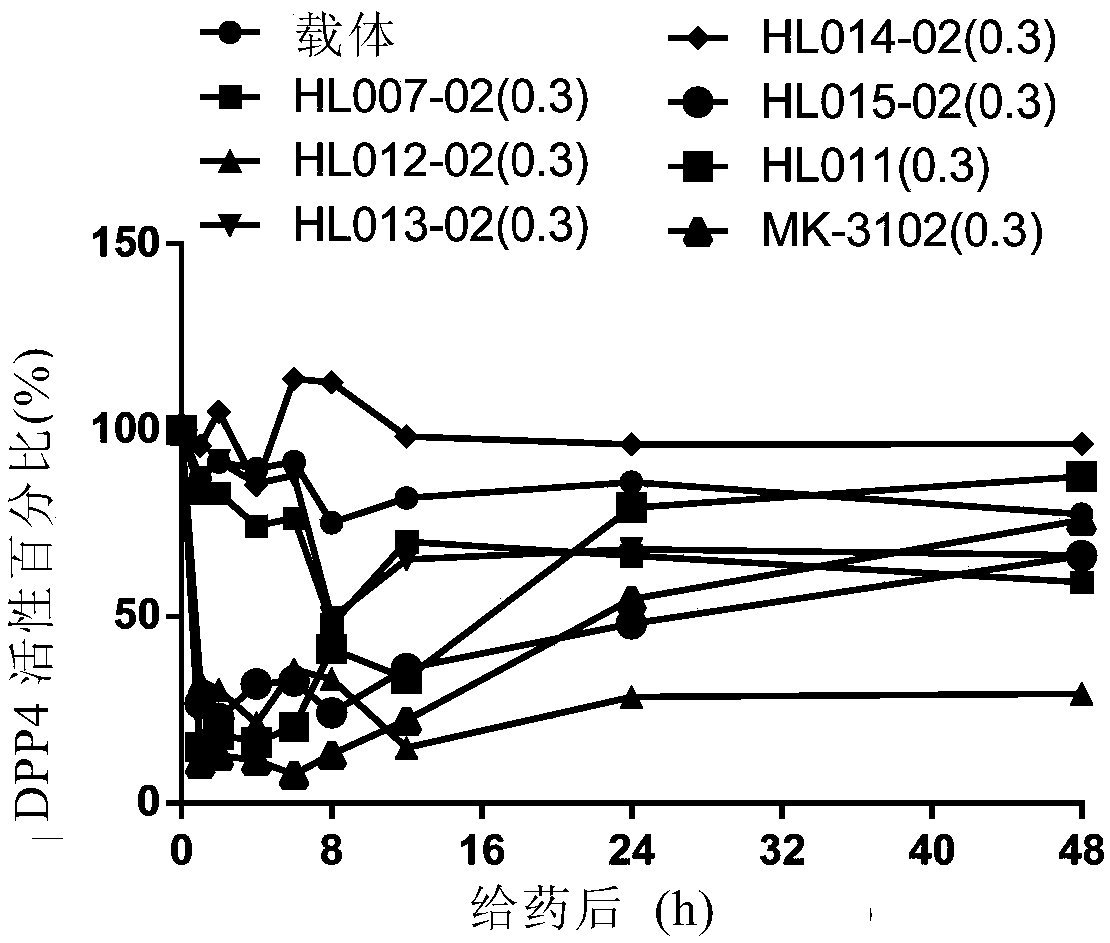
[0051] Embodiment 2. In vivo long-acting activity experiment (0.3mg / kg)
[0052] Instrument: microplate reader (PerkinElmer, USA).
[0053] Materials: Human recombinant DPP-4, which was expressed in insect cells by using the baculovirus expression system Bac-to-Bac (purchased from GIBCO) in our laboratory according to conventional experimental techniques; the substrate Ala-Pro-AMC was obtained by Synthesized by Jill Biochemical (Shanghai) Company.
[0054] Principle of activity test: DPP-4, DPP-8 and DPP-9 can specifically hydrolyze the substrate Ala-Pro-AMC to generate product AMC, AMC is excited by 355nm ultraviolet light to produce 460nm emission light, dynamic measurement unit time 460nm wavelength The fluorescence value changes linearly at the position, and the DPP-4 activity is calculated.
[0055] Sample treatment: The sample was dissolved in DMSO and stored at low temperature. The concentration of DMSO in the final system was controlled within the range that did not ...
Embodiment 3
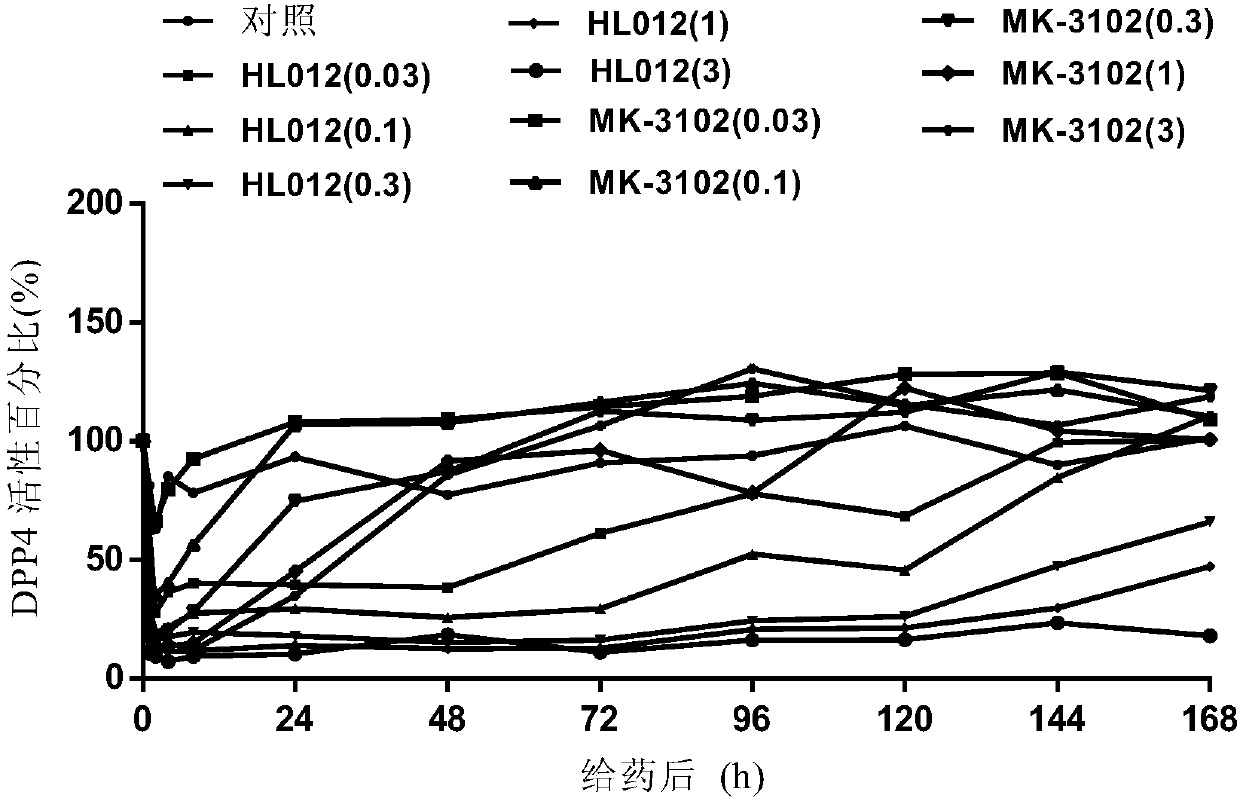
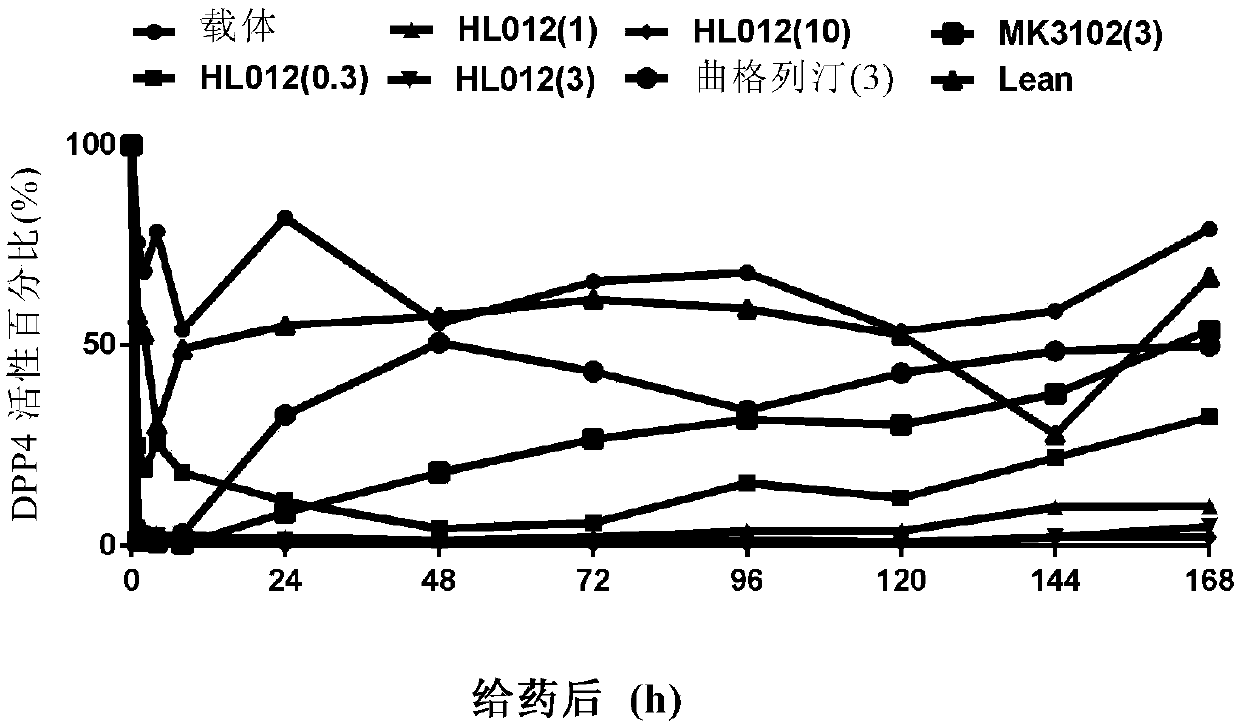
[0060] Example 3. DPP-4 Activity Study in Normal ICR Mice (Dose Dependency)
[0061] Animal: ICR mouse (8-10 weeks old, sex: male, body weight 25g-30g, purchased from Shanghai Slack Experimental Animal Center).
[0062] Steps: ICR mice were starved for 2h, orally administered test compound (0.03, 0.1, 0.3, 1 and 3mg / kg), positive drug MK-3102 (0.03, 0.1, 0.3, 1 and 3mg / kg); As a blank control, before oral administration, 1h, 2h, 4h, 8h, 24h, 48h, 72h, 96h, 120h, 144h and 168h after oral administration, blood was taken from the mouse orbital plexus vein, and added to an Eppendorf tube (Qizhong Industrial Co., Ltd. (Shanghai) Co., Ltd.), the serum was taken after centrifugation, and the DPP-4 activity was determined.
[0063] Results: Within 48 hours after a single administration of HL012, HL012 can significantly inhibit the DPP-4 activity of normal ICR in vivo, and presents a significant dose-dependent manner, in which HL012 0.3mg / kg and 1mg / kg DPP-4 within 2 hours after admin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com