Amylin compatible polypeptide and application thereof
A technology for affinity and peptides, applied in the field of Amylin affinity peptides and its applications, can solve the problems of lack of affinity inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
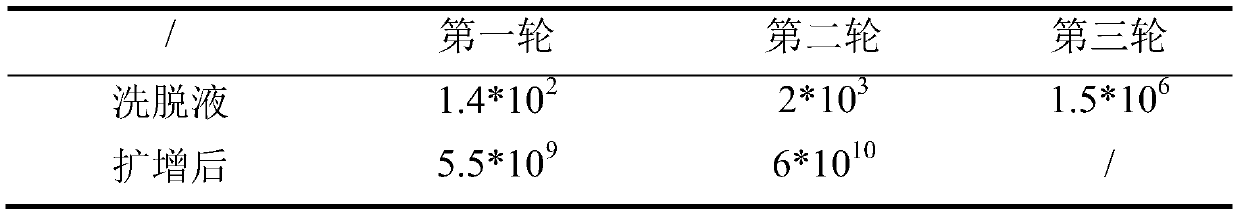
[0024] The screening of the affinity polypeptide of embodiment 1.Amylin
[0025] 1. Screening of affinity peptides for Amylin
[0026] ① Preparation of target and activation of host bacteria:
[0027] a. Streak inoculate the strain ER2738 preserved in glycerol on the LB-Tet plate, and culture it upside down at 37°C overnight. Store in the dark at 4°C after sealing with parafilm.
[0028] b. Prepare 6 small sterilized polystyrene Petri dishes, add 1.5mL of 100μg / mL streptavidin solution (dissolved in 0.1M NaHCO pH 8.6 3 ), and rotate well to wet the surface.
[0029] c. Incubate overnight at 4°C with slight shaking in a humidified container, and store at 4°C for later use.
[0030] d. Pick ER2738 monoclonal clone from the LB-Tet plate and inoculate it into 20 mL LB liquid medium, and culture it in a 250 mL Erlenmeyer flask at 37°C with vigorous shaking to the pre-logarithmic stage.
[0031] e. Take a well-coated board, pour out the coating solution, and shake the board vig...
Embodiment 2
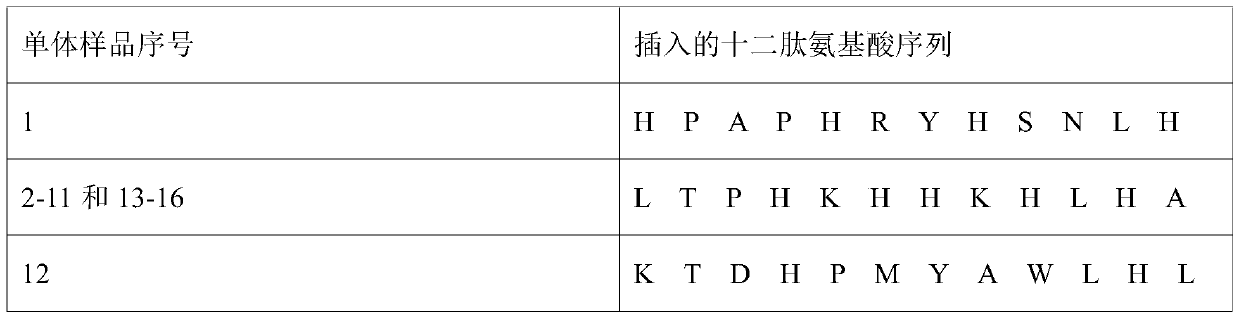
[0096] Example 2.LA12 has an inhibitory effect on the process of Amylin aggregation to form fibers
[0097] Molecular simulation predicts that the binding site between Amylin and LA12 contains the hotspot sequence where Amylin aggregates to form a β-sheet, and isothermal titration calorimetry experiments show that Amylin has a strong affinity with LA12. Based on this, LA12 can be used in the research of Amylin inhibitors .
[0098] ThT fluorescence detection:
[0099] Amylin treatment: Dissolve the lyophilized powder in hexafluoroisopropanol to prepare a 1mg / mL peptide stock solution, sonicate in a water bath for 5min, dry it in vacuum, and redissolve it in 10mM Tris-HCl buffer at pH 7.3 , diluted to 30μM (final concentration), dispensed into each EP tube, and shaken at 220rpm / min at 37°C for about 24h. The sampling time points were 0h, 2h, 8h, 12h, 18h, and 24h. Figure 8 . The analysis shows that the entire growth process of Amylin can be clearly divided into two stages: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com