Aromatic rose ether perfume synthesis method
A technology of aromatic rose ether and synthesis method, which is applied in directions such as organic chemistry, can solve problems such as unfavorable industrial production, equipment corrosion, low reaction yield and the like, and achieves the effect of improving selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
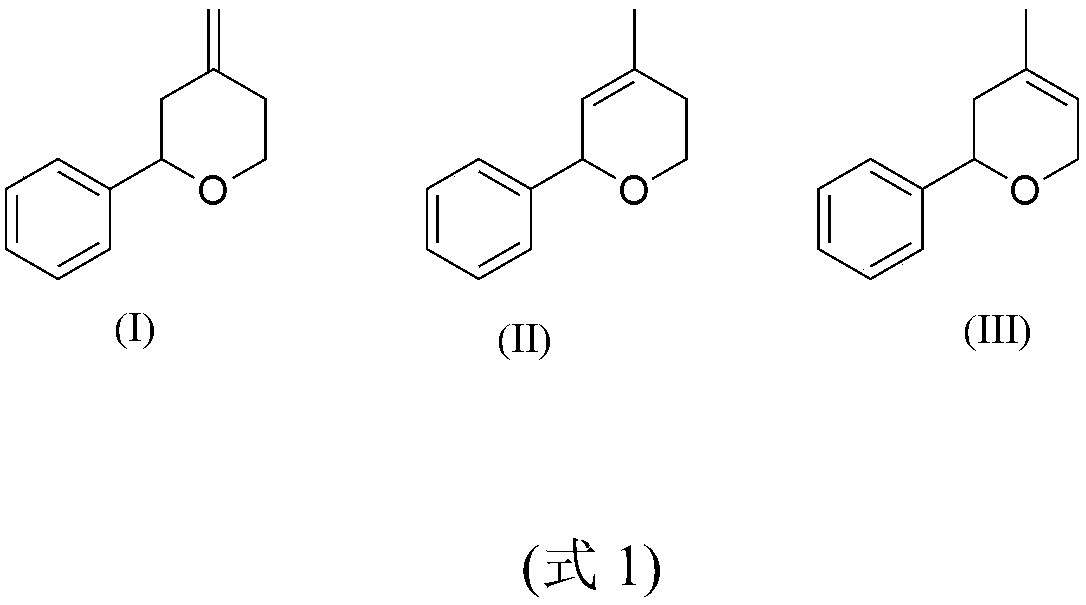
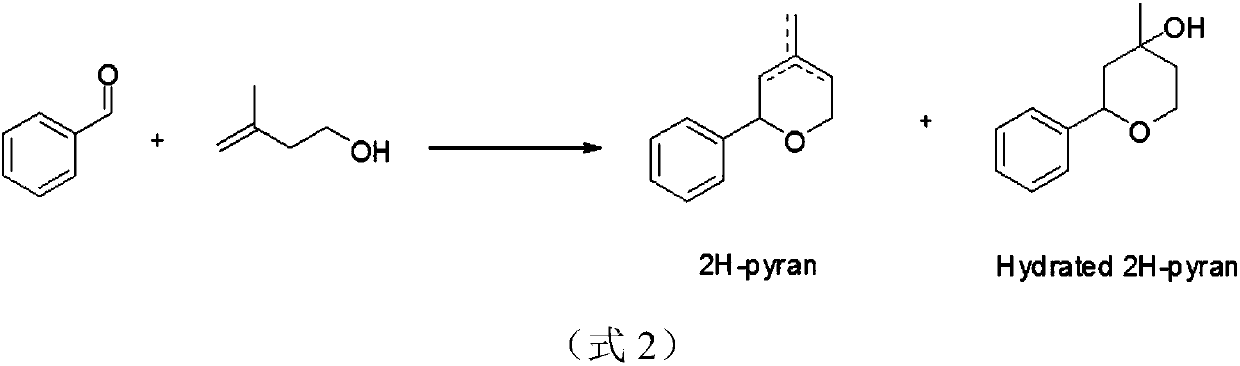
[0026] 387 g (3.65 mol) of benzaldehyde, 400 ml of toluene and 0.73 g of PPTS were added to a one-liter jacketed reactor equipped with mechanical stirring, a Dean-Stark trap and a dropping funnel. (3.0mmol) was heated to reflux for one hour, and 300 grams (3.49mol) of isopentenol was added dropwise over 10 hours. After the dropwise addition, the reaction was continued until the water separator stopped collecting the water generated by the reaction, and cooled to room temperature. Add 200 ml of 5% acetic acid aqueous solution and 200 ml of water to wash successively. After recovering the solvent under reduced pressure, the crude product was purified by rectification to obtain 480 g of dihydropyran product (83-84° C. / 1 mmHg, molar yield 79%).
Embodiment 2
[0028] Into a one liter jacketed reactor equipped with mechanical stirring, Dean-Stark trap and dropping funnel were charged 500 g (4.72 mol) of benzaldehyde, 500 ml of xylene and 0.85 g of PPTS (3.4 mmol). One hour after heating to reflux, 300 grams (3.49 mol) of prenol was added dropwise over 12 hours. After the dropwise addition, the reflux was continued until the water separator stopped collecting the water generated by the reaction, and cooled to room temperature. Add 200 ml of 5% acetic acid aqueous solution and 200 ml of water to wash successively. After recovering the solvent under reduced pressure, the crude product was purified by rectification to obtain 460 g of dihydropyran product (83-84° C. / 1 mmHg, molar yield 75%).
Embodiment 3
[0030] Into a one liter jacketed reactor equipped with mechanical stirring, Dean-Stark trap and dropping funnel were added 387 g (3.65 mol) of benzaldehyde, 180 ml of toluene and 0.35 g of ferric sulfate (0.87 mmol). One hour after heating to reflux, 300 g (3.49 mol) of isopentenol was added dropwise over 8 hours. After the dropwise addition, reflux was continued for one hour, and then heating was stopped and cooled to room temperature. Add 200 ml of 5% acetic acid aqueous solution and 200 ml of water to wash successively. The solvent was recovered under reduced pressure, and the crude product was rectified under reduced pressure to obtain 540 grams of dihydropyran product (83-84°C / 1mmHg, molar yield 89%.)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com