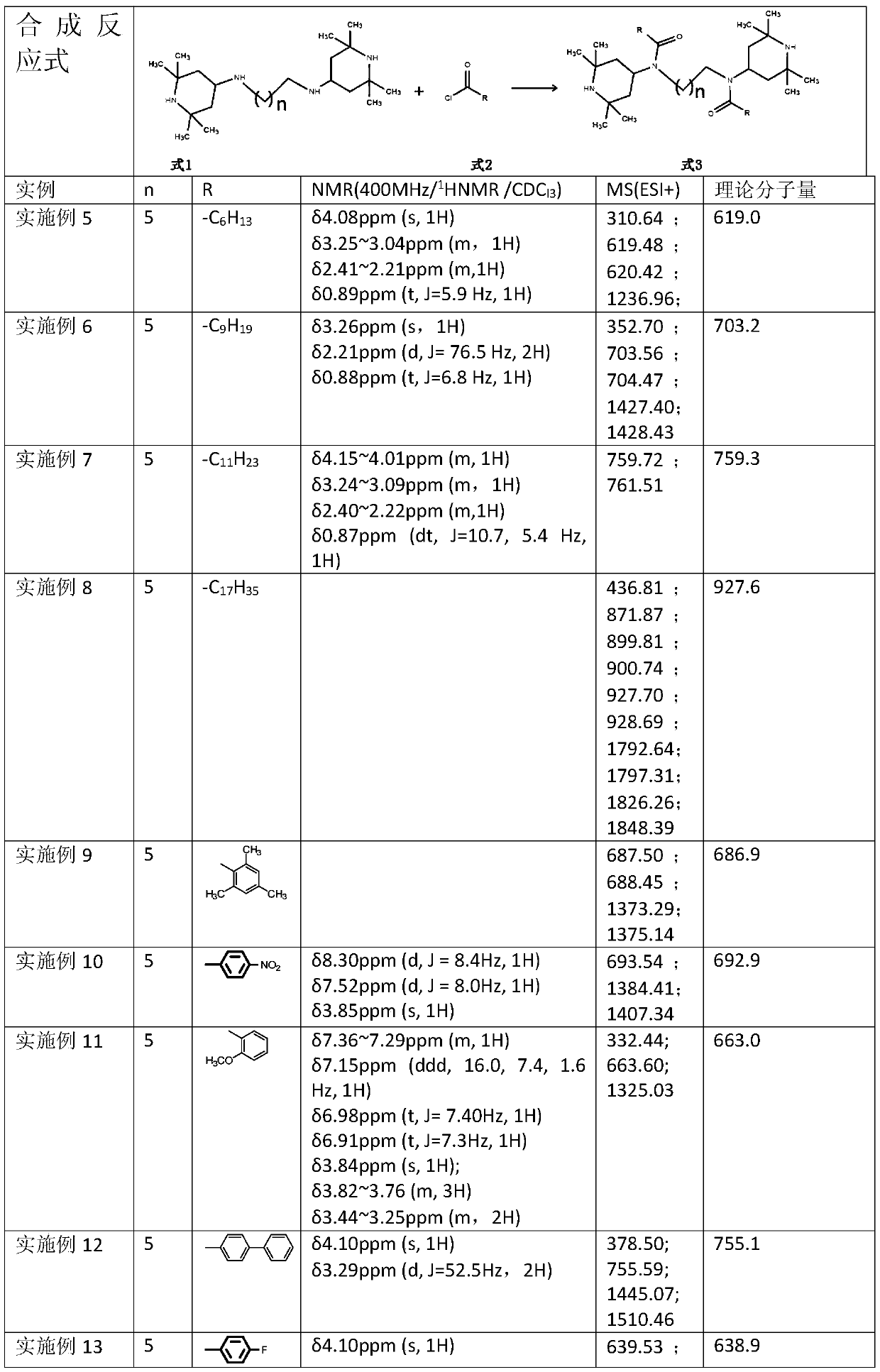
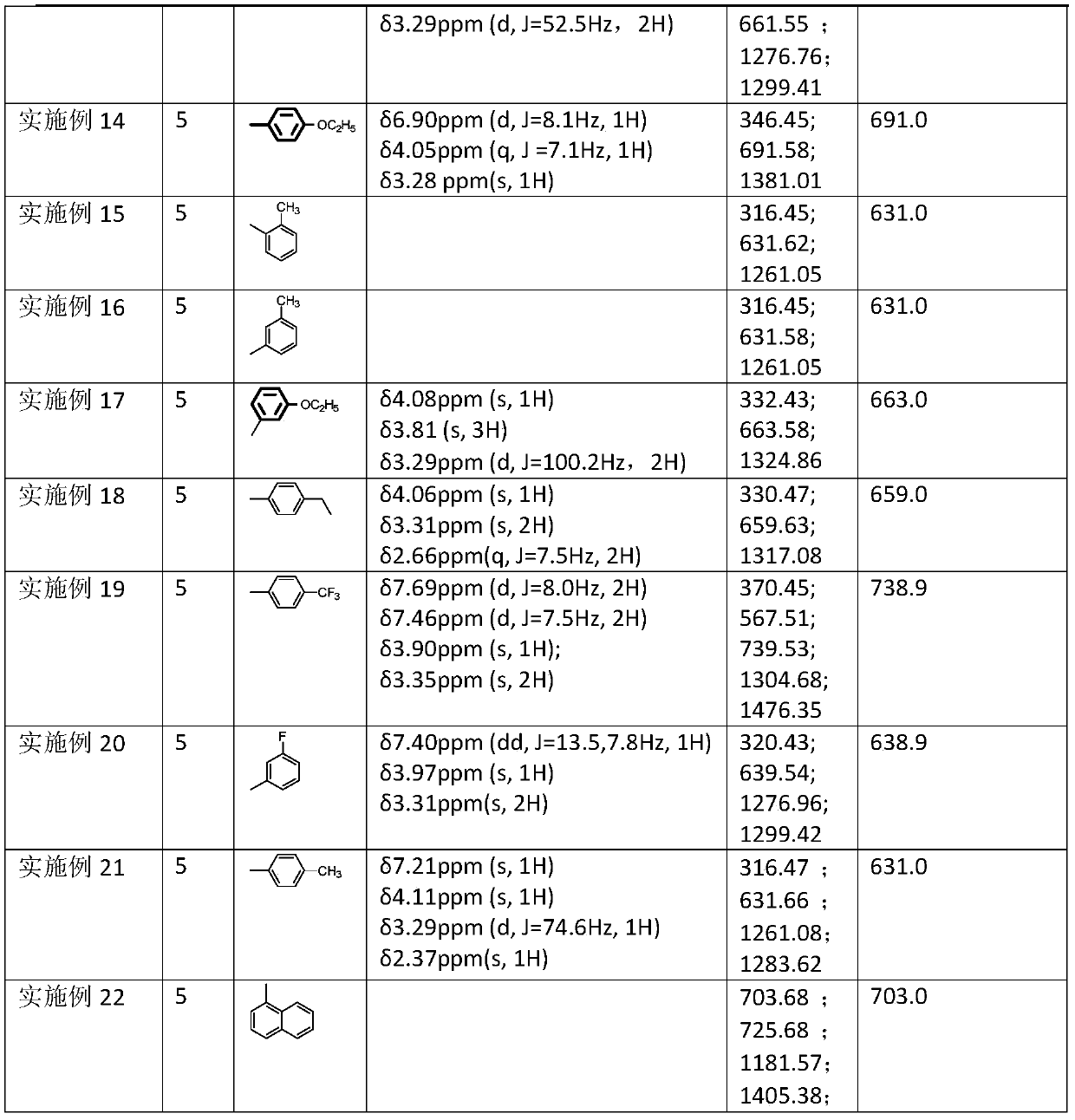
Novel hindered amine light stabilizers, and preparation method and applications thereof
A technology of light stabilizers and hindered amines, applied in the field of light stabilizers and their synthesis, to achieve the effects of high yield, improved physical properties, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0022] 3.97g (10.06mmol) hexamethylenediaminepiperidine (the situation where n of the compound of formula 1 is 5) is dissolved in 20ml dry solvent (DCM, or THF or ethyl acetate or MTBE, or acetone), add 21.12mmol acid-binding agent ( Anhydrous potassium carbonate or NaOH or Na2CO3 or NEt3), under the protection of N2, the temperature of the ice-salt bath is reduced to -10°C, and 2.82g of benzoyl chloride (when the compound R of formula 2 is -Ph) is added dropwise, and the dropwise addition is completed in about 30 minutes. Stir at zero for 1 h, allow to warm up to room temperature and stir for 6 h, filter, recover the solvent under reduced pressure at 40°C, recrystallize to obtain 5.8 g of white solid, yield: 96%.
[0023] 1 H-NMR / CDCl 3 / 400MHz: δ7.346~7.423ppm(t,J=5.1Hz,5H),3.150~3.343ppm(s,2H),4.026ppm(s,1H)
[0024] MS (ESI+): theoretical 602.9; measured 302.40, 603.37, 604.31, 1205.00, 1227.16, 1229.12.
example 2
[0026] 3.97g (10.06mmol) hexamethylenediamine piperidine is dissolved (the situation that formula 1 compound n is 5) in 20ml dry solvent (DME or THF or acetone or ethyl acetate or toluene or MTBE), add 21.12mmol acid-binding agent ( Anhydrous NaOH or K2CO3 or NEt3 or pyridine or Na2CO3), the temperature of the ice-salt bath under N2 protection is down to-10 ℃, and 3.25g o-fluorobenzoyl chloride (compound R of formula 2 is ) dropwise, stirred at zero for 1 h, allowed to warm up to room temperature and stirred for 10 h, TLC followed the reaction until the reaction was complete and filtered, recovered the solvent under reduced pressure at 40°C, recrystallized to obtain 6 g of off-white solid, yield: 94%. 1 H-NMR / CDCl 3 / 400MHz: δ7.37ppm (dd, J = 13.3, 5.9Hz, 1H), 7.34~7.28ppm (m, 1H), 7.20ppm (t, J = 7.5Hz, 1H), 7.11ppm (t, J = 8.8 Hz, 1H), δ3.86ppm (t, J=11.9Hz, 1H), δ3.36ppm (d, J=54.4Hz, 2H)
[0027] MS (ESI+): theoretical 638.9; measured: 320.39, 639.54, 1276.94.
example 3
[0029] 3.97g (10.06mmol) hexamethylenediamine piperidine is dissolved (the situation that formula 1 compound n is 5) in 20ml dry solvent (MTBE or THF or DME or acetone or ethyl acetate), add 21.12mmol acid-binding agent (NaCO Or K2CO3 or NaOH), under N2 protection, the temperature of the ice-salt bath is down to-10°C, and slowly add 3.5g p-methoxybenzoyl chloride dropwise (compound R of formula 2 is In the case of the reaction), the dropwise addition was completed in about 30 minutes, stirred at zero for 1 hour, allowed to rise naturally to room temperature and stirred for 6-18 hours, TLC followed the reaction until the reaction was complete and filtered, the solvent was recovered under reduced pressure at 40°C, recrystallized to obtain 6.5 g of a white solid, and The rate is 98%.
[0030] 1 H-NMR / CDCl 3 / 400MHz: δ6.92ppm (d, J = 8.5Hz, 1H), 3.83ppm (s, 1H), 3.32ppm (s, 1H)
[0031] MS(ESI+): Theory 663.0; Measured: 332.48, 663.63, 1325.14, 1347.57
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com