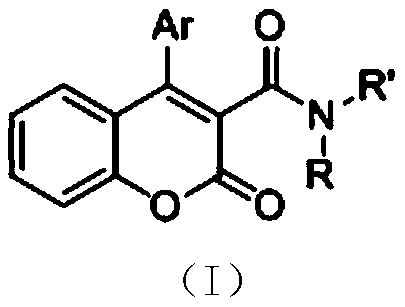
3-Amide-4-phenylcoumarin and preparation method thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A kind of synthetic method of 3-cyclohexyl amido-4-phenylcoumarin, comprises the following steps:
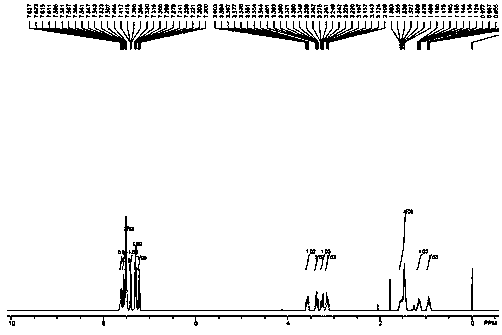
[0035] Under a nitrogen atmosphere, add 222mg of phenylpropiolate, 314mg of carbonyl (1-piperidinyl) acetic acid, 540mg of potassium persulfate, 17mg of silver nitrate, 2.5mL of water and 2.5mL of acetonitrile into a 25mL three-necked flask, and then The bottle was placed in an oil bath at 60°C, and the reaction was stirred at a magnetic stirring speed of 800r / min. During the reaction, the concentration of phenylpropiolate in the reaction liquid was monitored by thin-layer chromatography, and the reaction liquid was detected after 12 hours of reaction. The reaction of phenylpropiolate is complete, that is, the reaction is ended, and then the reaction solution is cooled to room temperature, filtered and concentrated to obtain 160 mg of 3-cyclohexylamide-4-phenylcoumarin in the form of white needles, with a yield of 60% .
[0036]
Embodiment 2
[0038] A kind of synthetic method of 3-cyclohexyl amido-4-phenylcoumarin, comprises the following steps:
[0039] Under a nitrogen atmosphere, add 222mg of phenylpropiolate, 314mg of carbonyl (1-piperidinyl) acetic acid, 540mg of potassium persulfate, 17mg of silver nitrate, 2.5mL of water and 2.5mL of acetonitrile into a 25mL three-necked flask, and then The bottle was placed in an oil bath at 100°C, and the reaction was stirred at a magnetic stirring speed of 800r / min. During the reaction, the concentration of phenylpropiolate in the reaction liquid was monitored by thin-layer chromatography, and the reaction liquid was detected after 12 hours of reaction. The reaction of the phenylpropiolate is complete, that is, the reaction is terminated, and then the reaction solution is cooled to room temperature, filtered, and concentrated to obtain 167 mg of white needle-like 3-amide 4-phenylcoumarin product with a yield of 50%.
Embodiment 3
[0041] A kind of synthetic method of 3-cyclohexyl amido-4-phenylcoumarin, comprises the following steps:
[0042] Under a nitrogen atmosphere, add 222mg of phenylpropiolate, 314mg of carbonyl (1-piperidinyl) acetic acid, 540mg of potassium persulfate, 17mg of silver nitrate, 2.5mL of water and 2.5mL of acetonitrile into a 25mL three-necked flask, and then The bottle was placed in an oil bath at 120°C, and the reaction was stirred at a magnetic stirring speed of 800r / min. During the reaction, the concentration of phenylpropiolate in the reaction liquid was monitored by thin-layer chromatography, and the reaction liquid was detected after 12 hours of reaction. The reaction of the phenylpropiolate is complete, that is, the reaction is terminated, and then the reaction solution is cooled to room temperature, filtered, and concentrated to obtain 163 mg of white needle-like 3-amide 4-phenylcoumarin product with a yield of 48%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com