Glycoconjugate and application thereof
A technology for glycoconjugates and compositions, applied in the field of glycoconjugates and their uses, can solve the problems such as no disclosure of serotype 4 capsular polysaccharide oxidation sites, no disclosure of glycoconjugates, etc., and achieve immunogenicity High, bactericidal effect enhanced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1 Preparation of S. pneumoniae serotype 4-CRM197 glycoconjugate using TEMPO / NIS oxidized capsular polysaccharide
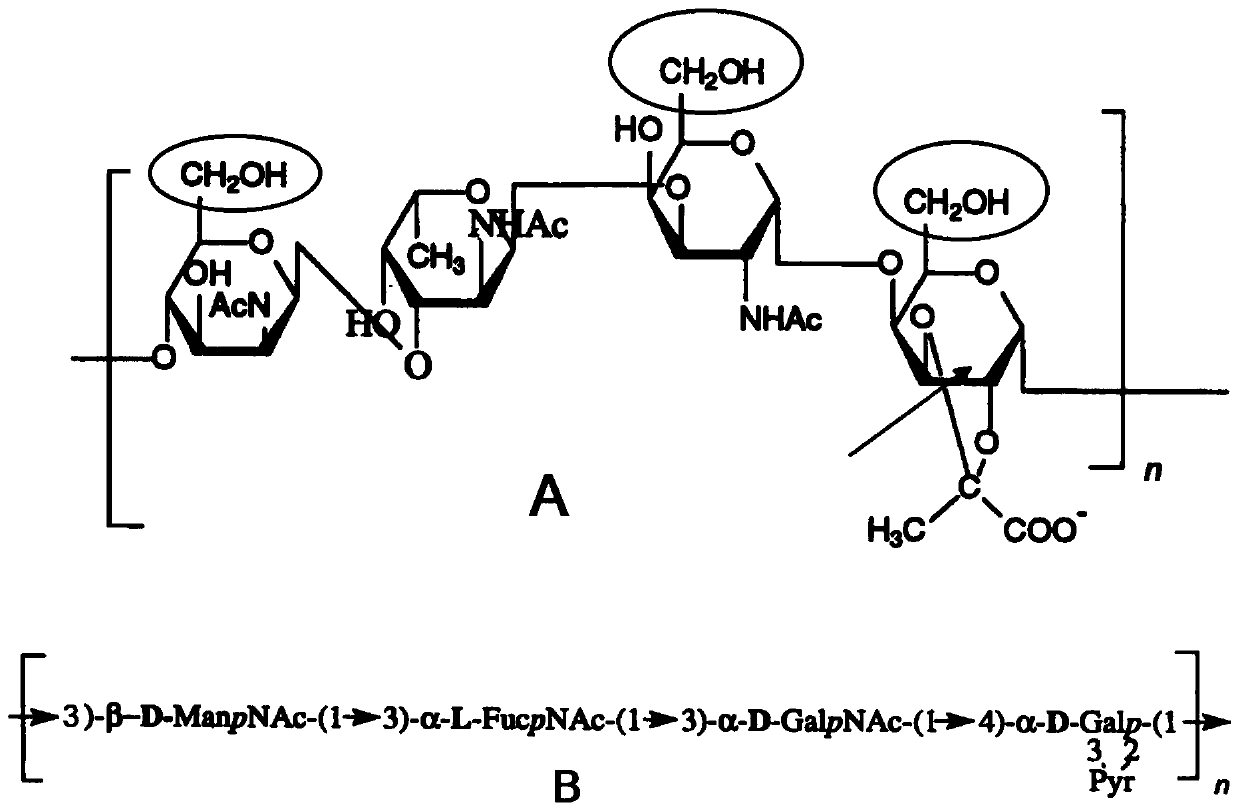
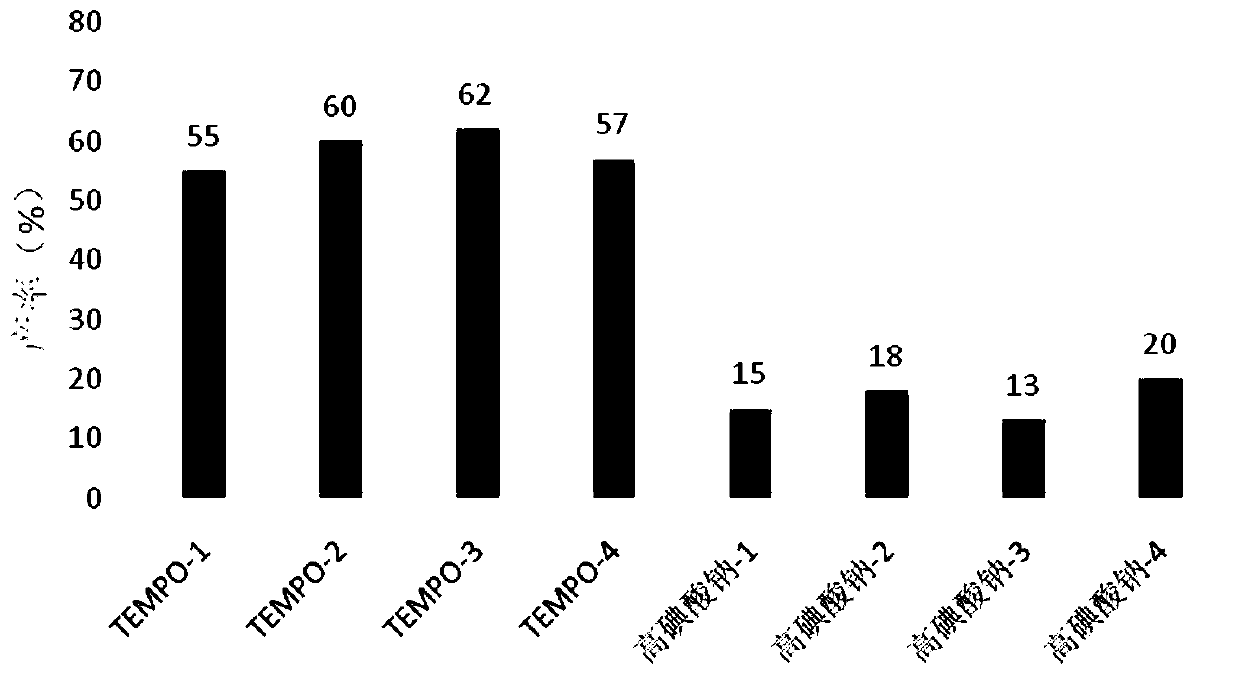
[0100] To improve the yield of serotype 4-CRM197 glycoconjugates, 2,2,6,6-tetramethyl-1-piperidinyloxy radical (TEMPO) and N-iodosuccinyl as co-oxidant were used The serotype 4-CRM197 glycoconjugate is prepared by reacting with the primary lysine amino group of the carrier protein by oxidizing the primary alcohol to an aldehyde group by using imine (NIS). NMR analysis indicated that the oxidation site was different from that of periodate-mediated oxidation. In the case of TEMPO-NIS oxidation, the oxidation sites are β-D-ManpNAc, α-D-GalpNAc and α-D-Galp (see figure 1 A elliptical part), while α-D-Galp is the predominant oxidation site when periodate is used (see figure 1 A arrow position).
[0101] The steps for preparing serotype 4-CRM197 glycoconjugates are as follows:
[0102] (1) using heating under acidic conditions to hydrolyze the serotyp...
Embodiment 2
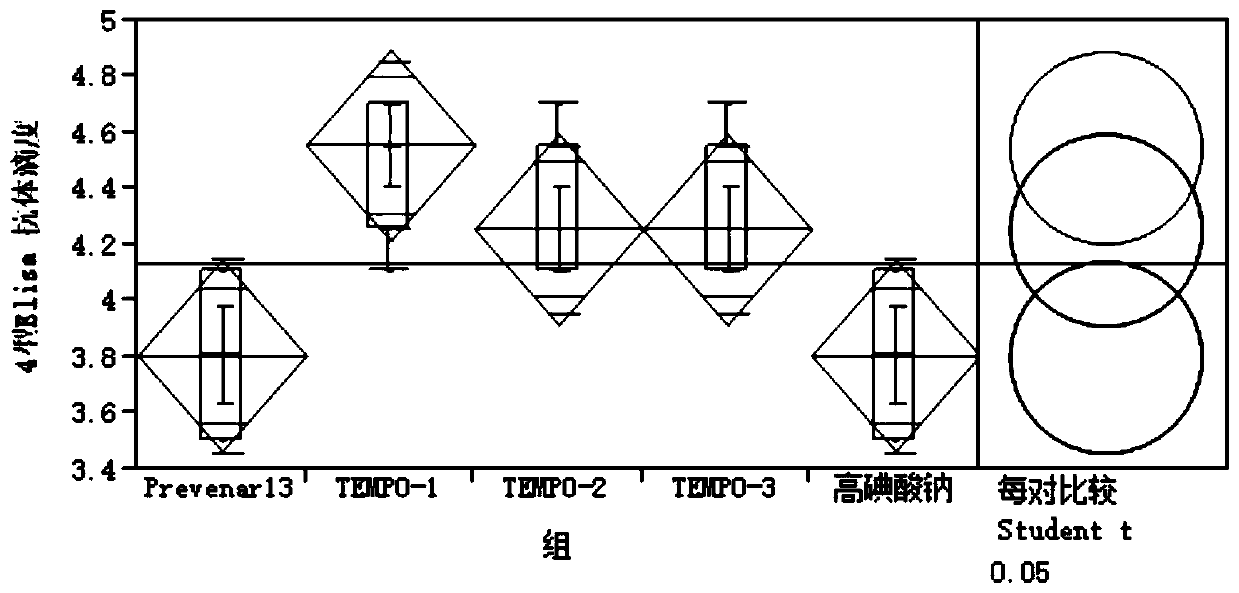
[0117] Example 2 Immunogenicity of Streptococcus pneumoniae serotype 4-CRM197 conjugate using TEMPO / NIS oxidation
[0118] According to the steps of preparing serotype 4-CRM197 glycoconjugates described in Example 1, the serotype 4-CRM197 conjugates of the test group were prepared, and the application effect of the conjugates was verified. Animal experiments were adopted, ordinary rabbits, about 2.5kg There are 10 groups in the experiment, 8 groups of experimental groups, 1 group of positive control, 1 group of negative control, 4 rabbits in each group, subcutaneous injection, immunization on 0, 14, 28 days respectively, the injection dose is human With half the dose, whole blood was collected from the neck vein 42 days after immunization and centrifuged at 8000 rpm for 6 min at 4°C to separate the serum and store it at -80°C. The specific test groups are shown in Table 3:
[0119] Elisa method: dilute type 4 capsular polysaccharide to the corresponding coating concentration w...
Embodiment 3
[0125] Example 3 Comparison of nuclear magnetic structures
[0126] Comparison of NMR structures of activated capsular polysaccharides, conjugates generated by periodate oxidation and activated capsular polysaccharides, conjugates generated by TEMPO / NIS oxidation (see Figure 5 ), indicating that the activated capsular polysaccharide of Streptococcus pneumoniae 4 was generated by TEMPO / NIS oxidation, and the capsular polysaccharide structure in the conjugate was closer to that of the purified capsular polysaccharide, and there was no significant difference. The conserved antigen α-D-Galp 2,3 There is no significant change in the pyruvic acid group at the position of the end group. The integral area statistics of the terminal proton and the pyruvate methyl group are used to calculate the molar ratio. The specific data are shown in Table 4. The results show that the activated capsular polysaccharide pyruvate protective group generated by TEMPO / NIS oxidation has been obtained. We...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com