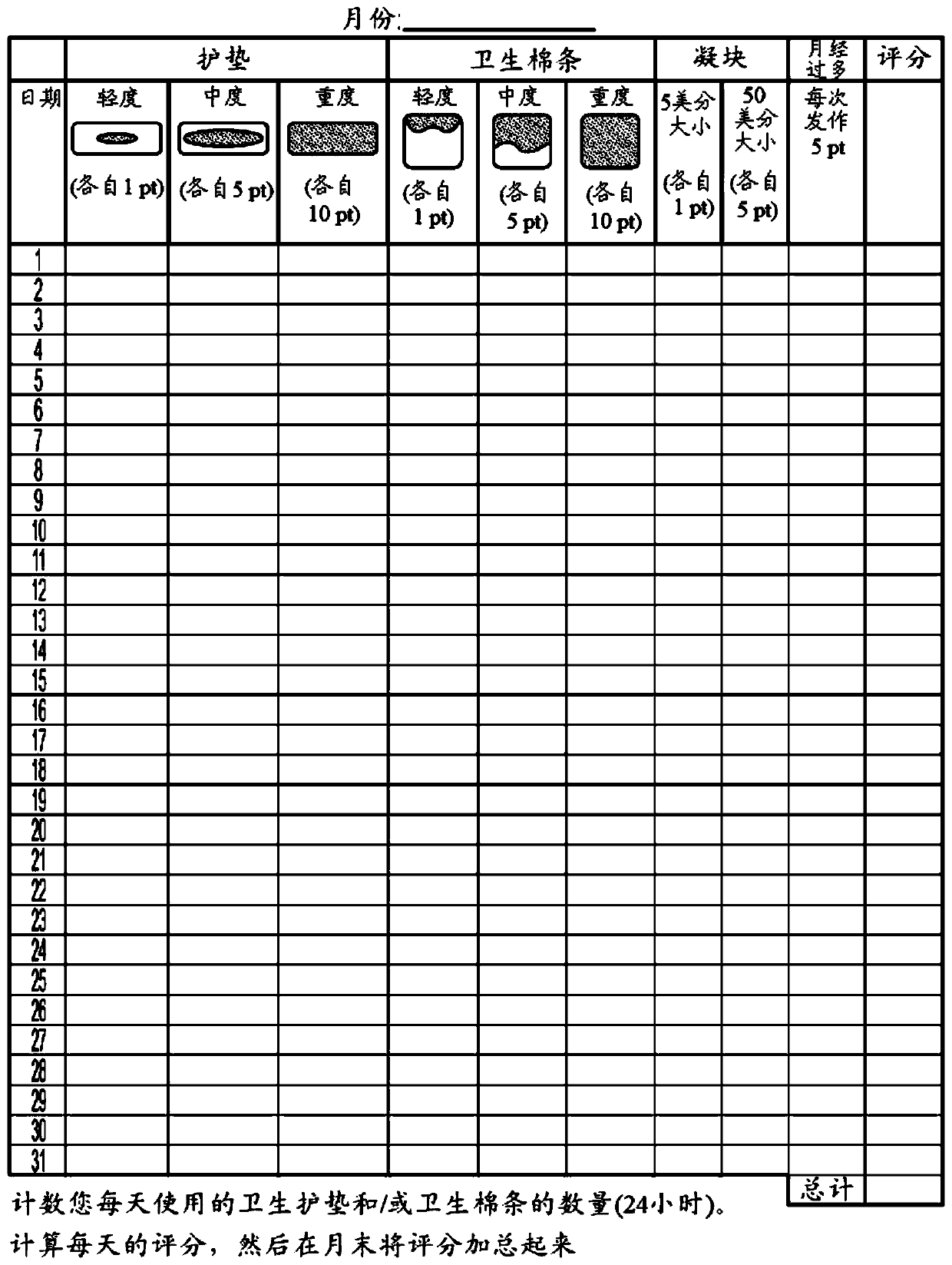
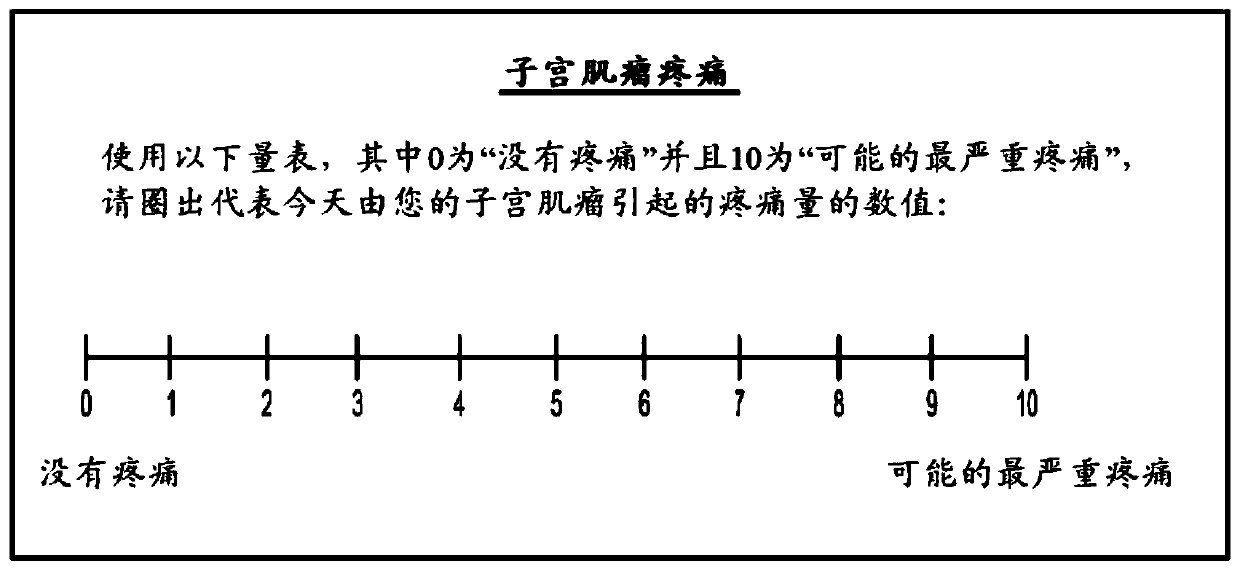
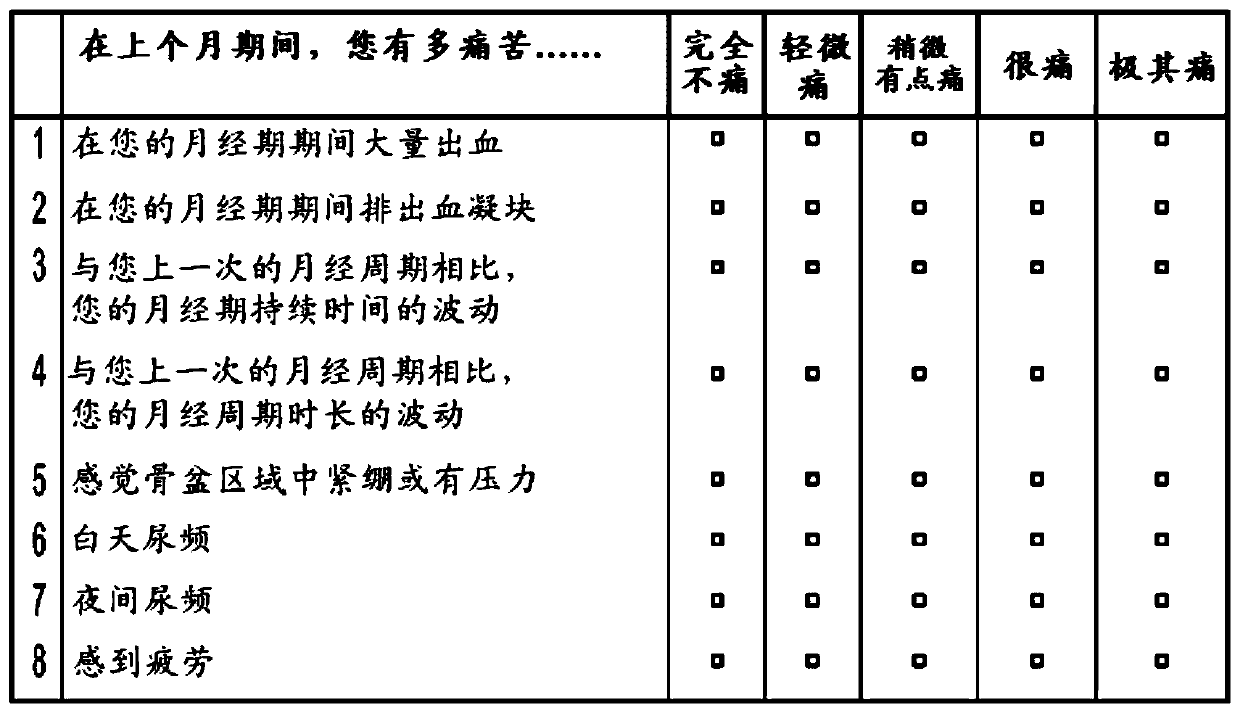
Methods of treating uterine fibroids and endometriosis
An endometrial, adenomyosis technique for use in the treatment of one or more side effects of gonadotropin-releasing hormone antagonist administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0665] Embodiment 1: preparation compound 1
[0666]
[0667] N-(4-(1-(2,6-difluorobenzyl)-3-(6-methoxy-3-pyridazinyl)-5-((methylamino)methyl)-2,4 -Dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl)phenyl)-N'-methoxyurea (150 mg, 0.259 mmol) in DMF (4ml), and iodomethane (0.010ml, 0.164mmol) was added thereto. The reaction mixture was stirred at room temperature for 1 hour, combined with aqueous sodium bicarbonate, and extracted with ethyl acetate. The organic layer was washed with brine, dried over magnesium sulfate and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: ethyl acetate / methanol=40 / 1), and recrystallized from dichloromethane / methanol / diethyl ether to give the title compound (17.3 mg, 17 %). 1 H-NMR (CDCl 3 )δ:2.15(6H,s),3.6-3.8(2H,m),3.82(3H,s),4.18(3H,s),5.35(2H),6.92(2H,t,J=8.2Hz), 7.12 (1H, d, J=8.8Hz), 7.2-7.65 (7H, m), 7.69 (1H, s).
Embodiment 2
[0668] Example 2: Preparation of Film Coated Tablets of Compound 1
[0669] By using the compound (40 mg) obtained in Example 1, mannitol (preferably D-mannitol) (122 mg), microcrystalline cellulose (40 mg), hydroxypropyl cellulose (6 mg), croscarmellose Sodium (10 mg), magnesium stearate (2 mg) and sufficient purified water to prepare film-coated tablets. Water is removed during processing. In a fluidized bed drying granulator (LAB-1, Powrex Corporation), the compound obtained in Example 1, D-mannitol, and microcrystalline cellulose were preheated and mixed, an aqueous solution of hydroxypropyl cellulose was sprayed, and the mixture was dried to obtain granular powder. To the obtained granulated powder, croscarmellose sodium and magnesium stearate were added, and they were mixed in a bag to obtain a mixed powder. The mixed powder has Tablets were compressed with a rotary tablet press (compact 10 tablet press, Kikusui Seisakusho Ltd.) with a ram to obtain tablet cores. ...
Embodiment 3
[0670] Example 3: Preparation of Film Coated Tablets of Compound 1
[0671] By using the compound (40 mg) obtained in Example 1, mannitol (including D-mannitol) (51 mg), sodium starch glycolate (Type A) (5 mg), hydroxypropylcellulose (3 mg), stearic acid Magnesium (1 mg) and sufficient purified water to prepare film-coated tablets. Water is removed during processing. In a fluidized bed drying granulator (LAB-1, Powrex Corporation), the compound obtained in Example 1, mannitol, and sodium starch glycolate were preheated and mixed, an aqueous solution of hydroxypropylcellulose was sprayed, and the mixture was dried to A granular powder was obtained. Magnesium stearate was added to the obtained granulated powder, and they were mixed in a bag to obtain a mixed powder. The mixed powder has Tablets were compressed with a rotary tablet press (compact 10 tablet press, Kikusui Seisakusho Ltd.) with a ram to obtain tablet cores. The tablet core was placed in a film coater (DRC-200...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com