Method for directly producing aluminum fluoride by fluorosilicic acid
A technology of aluminum fluoride and fluorosilicic acid, used in aluminum fluoride, aluminum halide, silicon oxide, etc., can solve the problems of flooding and wrapping, and achieve the effects of convenient measurement, low aluminum content, and high Al recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
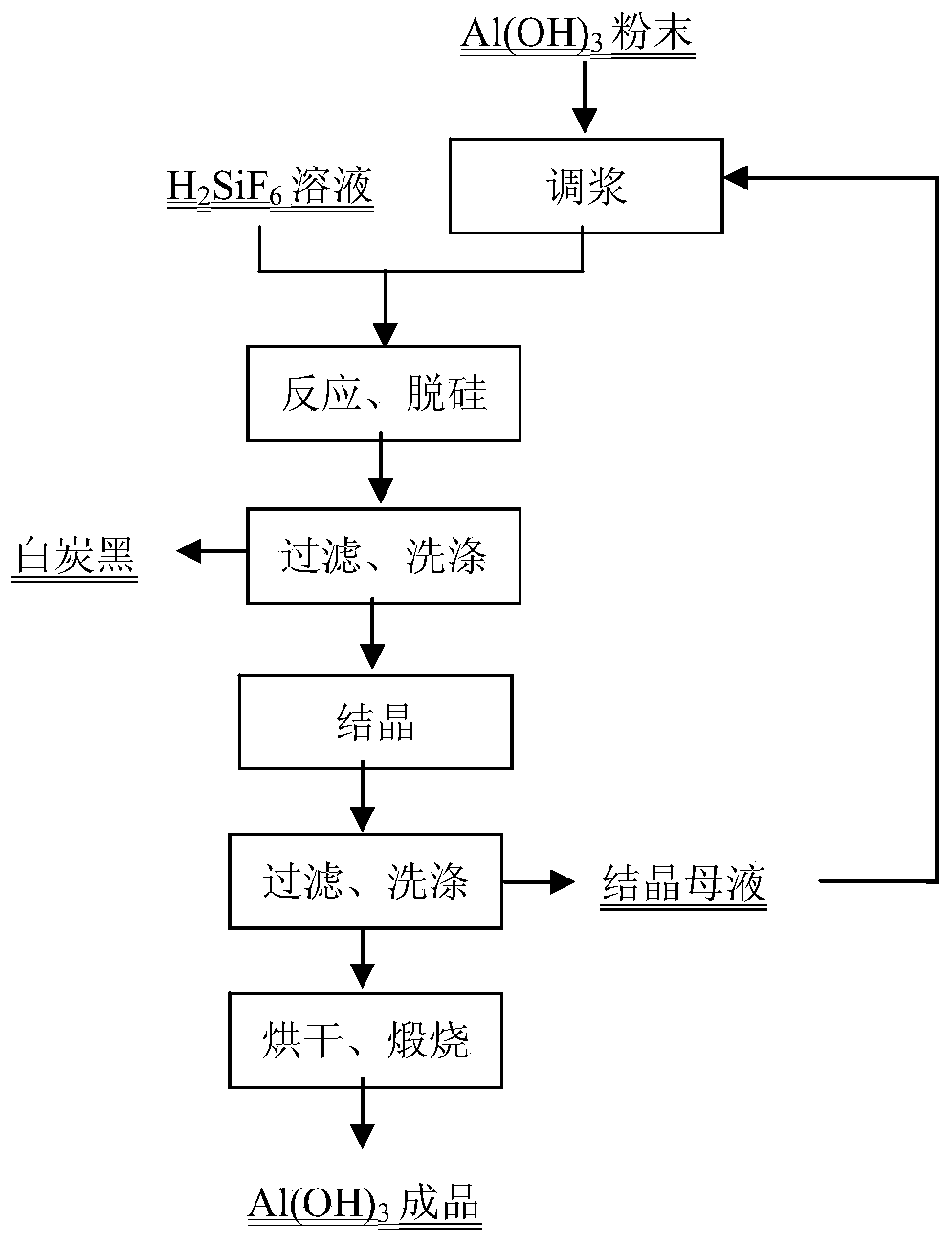
[0024] 1) Pre-slurry the aluminum fluoride crystallization filtrate and aluminum hydroxide at a mass ratio of 1.5:1 to obtain aluminum hydroxide slurry, and the slurry temperature is 48°C;
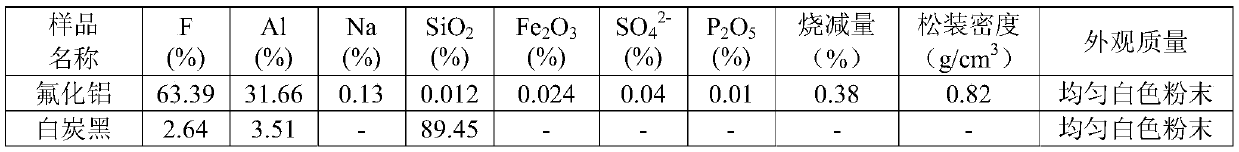
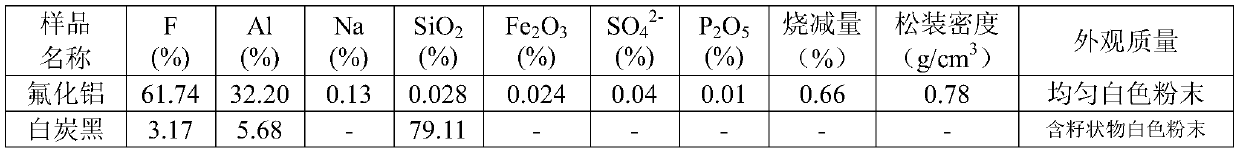
[0025] 2) Pump the measured aluminum hydroxide slurry into the measured fluosilicic acid solution preheated to 85°C for reaction, the excess of fluosilicic acid is 3%, the feeding time is 4min, and the fluosilicic acid in the fluosilicic acid solution ( h 2 SiF 6 ) mass percent 20.73%, F / Si molar ratio 5.3, P 2 o 5 The mass percentage is 0.007%, the reaction temperature is 93°C, and the reaction time (after feeding) is 17min;
[0026] 3) After the reacted slurry is filtered and washed, wet silica filter cake, filtrate and washing water are obtained;
[0027] 4) After the filtrate and washing water are combined, crystallize at 95°C, and the crystallization time is 4.5h;
[0028] 5) After crystallization, cool down the slurry to 60°C, filter and wash to obtain wet aluminum fluoride and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com