Catalyst system for preparing block polymer and method for catalytic synthesis of block polymer
A technology of block polymer and catalytic system, which is applied in the production of bulk chemicals, can solve the problems of waste of energy and increased cost, and achieve the effect of improving the possibility of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
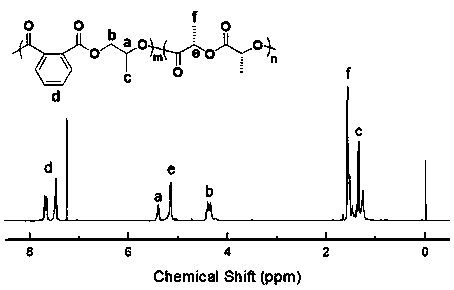
[0022] According to the molar ratio of 1:1, take N-methylimidazole and halogenated alkyl alcohol respectively, at a temperature of 50°C, in N 2 After reacting N-methylimidazole with halogenated alkanes for 24 h during protection, it was purified with acetonitrile and ether, and dried in vacuum to obtain a catalytic system (ionic liquid) for the preparation of block polymers. By comparing with published papers ( Angew. Chem. 2018, 130, 17130–17134), reflecting the effectiveness of the ionic liquid catalytic system prepared in Example 1 for the block copolymerization of cyclic anhydrides, epoxides and lactides.
[0023] Add 0.2 g of ionic liquid, 3 g of phthalic anhydride and 3 g of lactide into the reaction flask, vacuumize for 25 min, then add 5 mL of tetrahydrofuran and 5 mL of propylene oxide, and react at 80 °C for 24 h; After the reaction was completed, the reaction system was dissolved with chloroform, and purified with methanol acidified with hydrochloric acid to obtai...
Embodiment 2
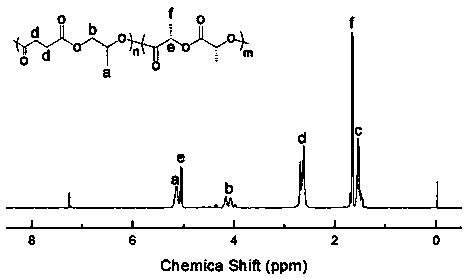
[0027] According to the molar ratio of 1:1.2, take N-methylimidazole and halogenated alkyl alcohol respectively, at a temperature of 70 ℃, in N 2 After reacting N-methylimidazole with halogenated alkanes for 10 hours during protection, purify with acetonitrile and ether, and dry in vacuum to obtain a catalytic system (ionic liquid) for preparing block polymers. By comparing with published papers ( Angew. Chem. 2018, 130, 17130–17134), reflecting the effectiveness of the ionic liquid catalytic system prepared in Example 2 for the block copolymerization of cyclic anhydrides, epoxides and lactides.
[0028] Add 0.05 g of ionic liquid, 2 g of succinic anhydride and 2 g of lactide into the reaction flask, vacuumize for 15 min, then add 5 mL of toluene and 4 mL of propylene oxide, and react at 90 °C for 12 h; the reaction is complete Afterwards, the reaction system was dissolved with chloroform, and purified with methanol acidified with hydrochloric acid to obtain a block polymer....
Embodiment 3
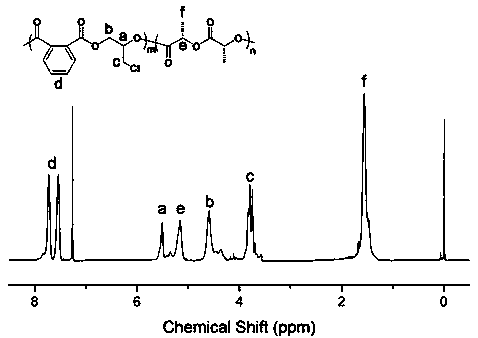
[0031] According to the molar ratio of 1:1.1, take N-methylimidazole and halogenated alkyl alcohol respectively, at a temperature of 60°C, in N 2 After reacting N-methylimidazole with halogenated alkanes for 17 h during protection, it was purified with acetonitrile and ether, and dried in vacuum to obtain a catalytic system (ionic liquid) for the preparation of block polymers. By comparing with published papers ( Angew. Chem. 2018, 130, 17130–17134) showed the effectiveness of the ionic liquid catalytic system prepared in Example 3 for the block copolymerization of cyclic anhydrides, epoxides and lactides.
[0032] Add 0.1 g of ionic liquid, 3 g of phthalic anhydride and 2 g of lactide into the reaction flask, vacuumize for 15 min, then add 5 mL of N, N-dimethylformamide and 5 mL of epichlorohydrin, The reaction was carried out at 70°C for 16 h; after the reaction was completed, the reaction system was dissolved in chloroform and purified with methanol acidified with hydroch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com