A kind of catalyst system for preparing aliphatic polyester and the method for using it to catalyze the synthesis of aliphatic polyester
A technology of aliphatic polyester and synthesis method, which is applied in the field of catalyst system for preparing aliphatic polyester, can solve the problems of expensive metal pollutants, metal catalyst pollution, hindering the application of polyester, etc., so as to avoid the problem of metal residue, catalyze High efficiency, improve the possible effect of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
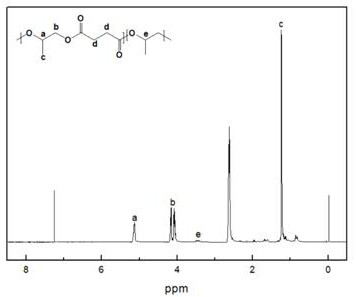
[0027] Add 0.02 g of DBU ionic liquid and 4.28 g of succinic anhydride into the reaction flask, vacuumize for 15 min, then add 5 mL of tetrahydrofuran and 3 mL of propylene oxide, and react at 120 °C for 10 h; Dissolve and settle with n-hexane as a precipitating agent to obtain aliphatic polyester.
[0028] The proton nuclear magnetic spectrum of the aliphatic polyester that embodiment 1 makes ( 1 H-NMR, CDCl 3 ) figure, such as figure 1 shown. The successful preparation of polyester can be seen from the figure. This demonstrates the effectiveness of the metal-free catalytic system of the present invention for the copolymerization of epoxides and cyclic anhydrides.
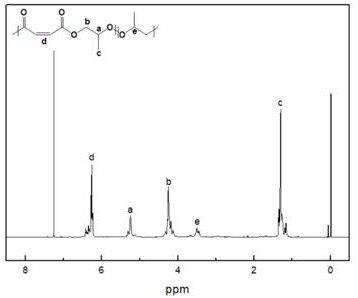
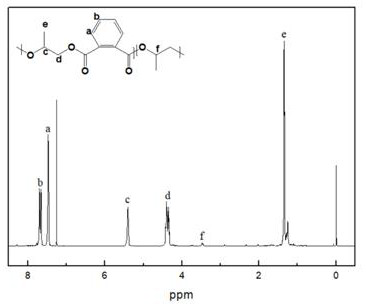
[0029] Figure 1 to Figure 5 The meaning of all coordinates in ppm is Chemical Shift.
Embodiment 2
[0031] Add 0.05 g of DBU-based ionic liquid and 4.214 g of maleic anhydride into the reaction flask, vacuumize for 20 min, then add 5 mL of tetrahydrofuran and 3 mL of propylene oxide, and react at 80 °C for 8 h. After the reaction is completed, the reaction system is dissolved with chloroform and settled with n-hexane as a sedimentation agent to obtain an aliphatic polyester.
[0032] The proton nuclear magnetic spectrum of the aliphatic polyester that embodiment 2 makes ( 1 H-NMR, CDCl 3 ) figure, such as figure 2 shown. The successful preparation of polyester can be seen from the figure. This demonstrates the effectiveness of the metal-free catalytic system of the present invention for the copolymerization of epoxides and cyclic anhydrides.
Embodiment 3
[0034] Add 0.02 g of DBU-based ionic liquid and 6.364 g of phthalic anhydride into the reaction flask, vacuumize for 30 min, then add 5 mL of tetrahydrofuran and 3 mL of propylene oxide, and react at 100 °C for 10 h. After the reaction is completed, the reaction system is dissolved with chloroform and settled with n-hexane as a sedimentation agent to obtain an aliphatic polyester.
[0035] The proton nuclear magnetic spectrum of the aliphatic polyester that embodiment 3 makes ( 1 H-NMR, CDCl 3 ) figure, such as image 3 shown. The successful preparation of polyester can be seen from the figure. This demonstrates the effectiveness of the metal-free catalytic system of the present invention for the copolymerization of epoxides and cyclic anhydrides.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com