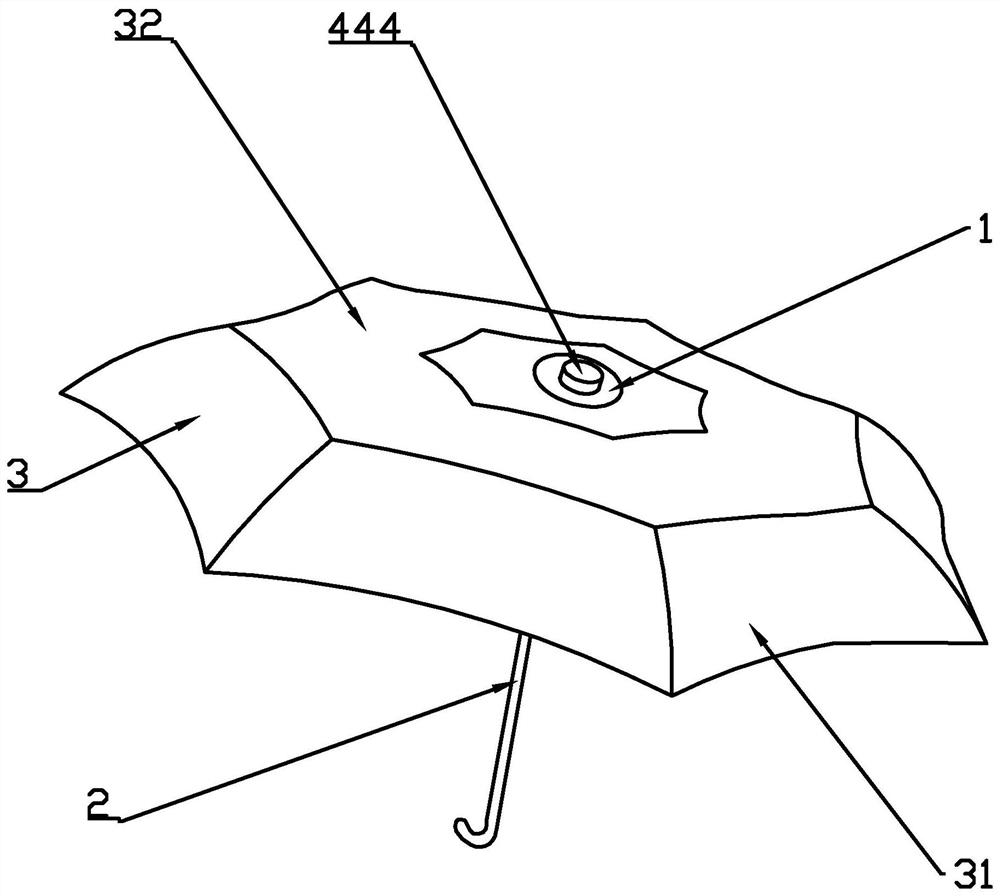
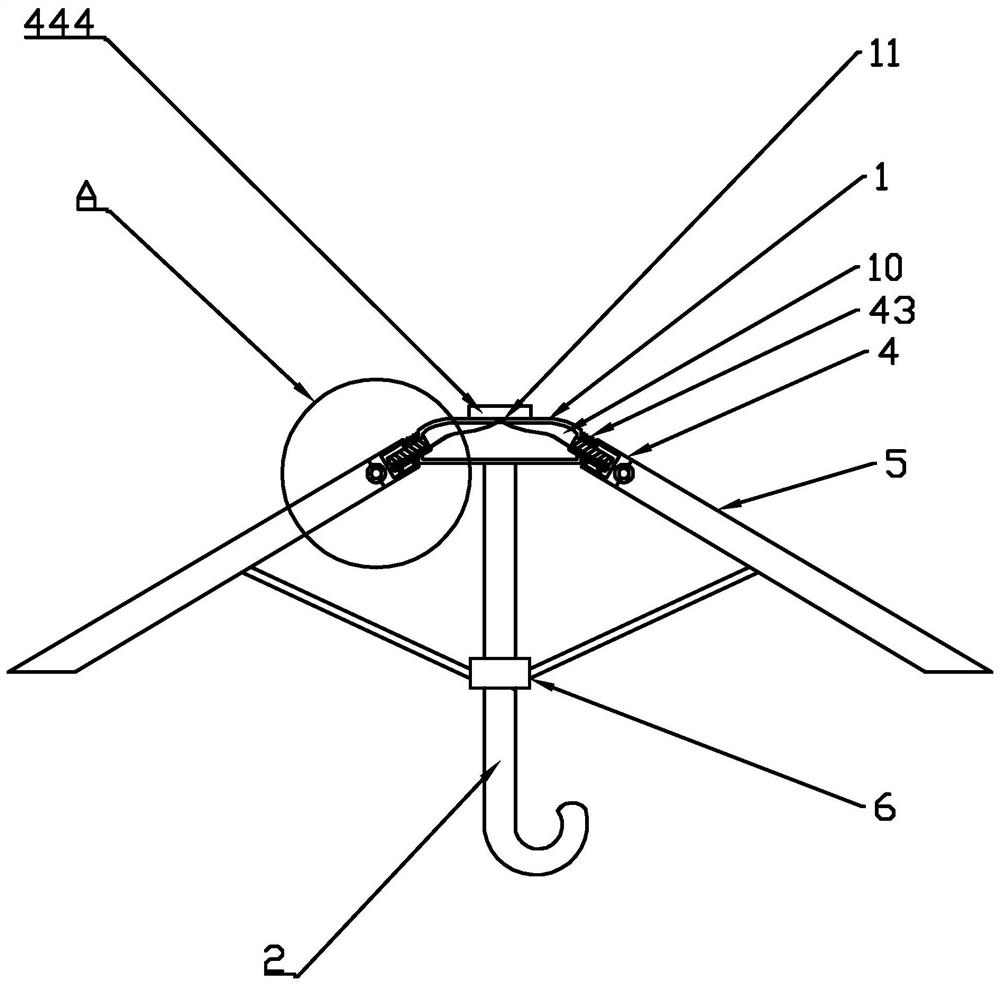
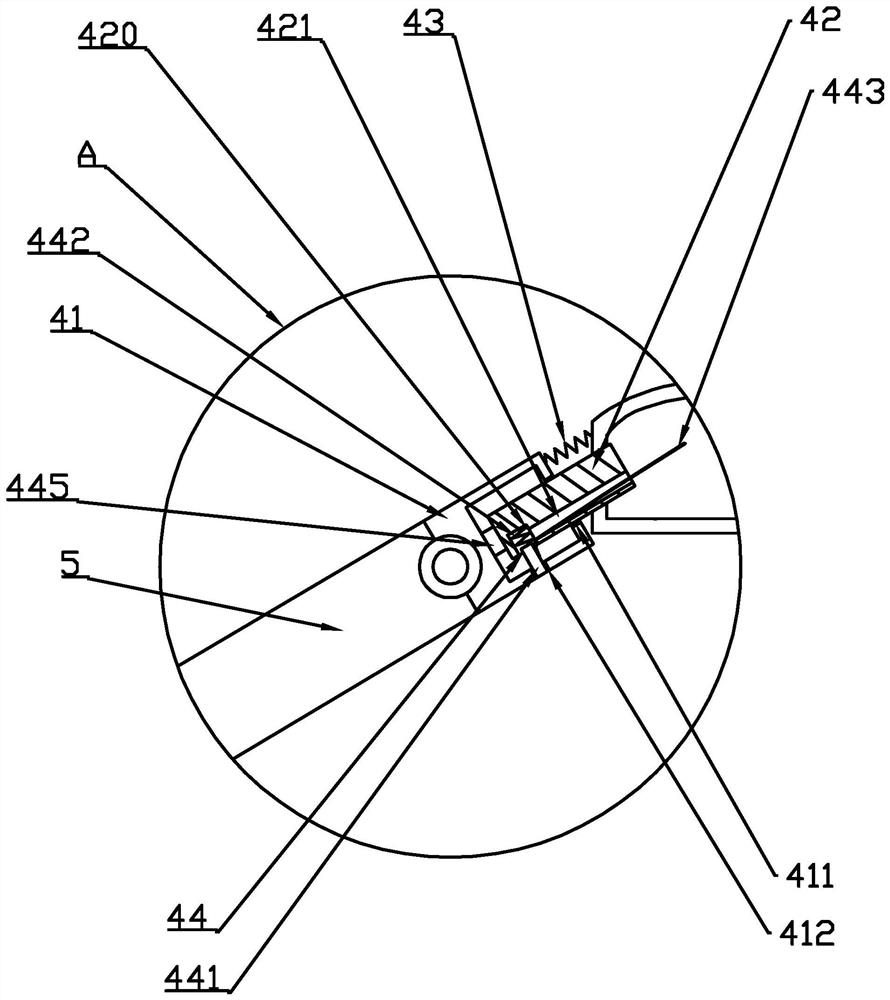
A convertible single and double umbrella
A two-person umbrella and umbrella handle technology, applied in the field of textile fabrics, can solve the problems of not being able to ensure that everyone is not exposed to the sun or rain, single function, discomfort, etc., and achieve the effect of simple structure, enlarged effective area, and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Weigh 13.1g (0.05mol) of dibromoneopentyl glycol, 10.1g (0.1mol) of triethylamine, and 100mL of acetone into a three-necked flask, slowly add 23.7g of (0.1mol) diphenylphosphinyl chloride, continue to react for 4 hours after the dropwise addition. After the reaction, the triethylamine salt was removed by filtration, the filtrate was distilled off under reduced pressure and dichloromethane was added, and washed three times with saturated saline and deionized water successively, and the organic phase was dried with anhydrous magnesium sulfate for 4 hours, then reduced The solvent was distilled off under reduced pressure to obtain diphenylphosphine dibromoneopentyl glycol ester. 1 H NMR (CDCl 3 ): 3.27(s,2H), 4.15(s,2H), 7.33(s,5H).
Embodiment 2
[0046] Weigh 8.65g (0.05mol) of dichloroneopentyl glycol, 10.1g (0.1mol) of triethylamine, and 100mL of acetone into a three-necked flask, slowly add 23.7g of (0.1mol) diphenylphosphinyl chloride, continue to react for 4 hours after the dropwise addition. After the reaction, the triethylamine salt was removed by filtration, the filtrate was distilled off under reduced pressure and dichloromethane was added, and washed three times with saturated saline and deionized water successively, and the organic phase was dried with anhydrous magnesium sulfate for 4 hours, then reduced The solvent was distilled off under high pressure to obtain diphenylphosphine dichloroneopentyl glycol ester. 1 H NMR (CDCl 3 ): 3.32(s,2H), 4.15(s,2H), 7.33(s,5H).
Embodiment 3
[0048] Weigh 7.75g (0.05mol) of 3-bromo-1,2-propanediol, 10.1g (0.1mol) of triethylamine, and 100mL of acetone into a three-necked flask, slowly dropwise add 23.7g (0.1mol) of diphenylphosphinyl chloride, continue to react for 4 hours after the dropwise addition. After the reaction, the triethylamine salt was removed by filtration, the filtrate was distilled off under reduced pressure and dichloromethane was added, and washed three times with saturated saline and deionized water successively, and the organic phase was dried with anhydrous magnesium sulfate for 4 hours, then reduced The solvent was distilled off under reduced pressure to obtain diphenylphosphinate 3-bromo-1,2-propanediol ester. 1 H NMR (CDCl 3 ): 3.49-3.82 (m, 2H), 4.32-4.61 (m, 2H), 7.33 (s, 5H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com