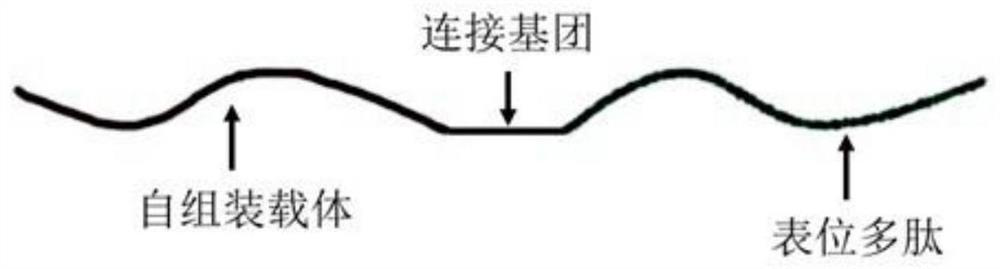
Co-assembled epitope vaccine and its application
An epitope vaccine and co-assembly technology, applied in the field of biomedical engineering, can solve the problems of ineffective stimulation of Th1 immune response, inability to meet tumor immunotherapy, weak immunogenicity, etc., to improve humoral immunity and enhance cellular immune response , strong specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation method of self-assembly epitope vaccine of the present invention is as follows:
[0036] Step 1. Preparation of epitope polypeptide bond
[0037] The epitope peptide and small molecule polypeptide carrier with a molar ratio of 1 to 3:1 are used as the epitope antigen and carrier, and the polypeptide carrier and epitope polypeptide are synthesized by solid-phase synthesis technology, and the amide bond, amino acid sequence or disulfide bond is selected as the connection Reactive groups such as amino, carboxyl, and sulfhydryl groups on epitope polypeptides and small molecule polypeptide carriers are prepared through amidation, sulfhydryl, and disulfide bond exchange reactions to prepare epitope polypeptide bonds, which are polypeptides containing antigenic epitopes sequence. If you choose the amide bond, it is the solid phase synthesis technique; if you choose the disulfide bond or the polypeptide carrier, it is the epitope peptide modified cysteine, the ...
Embodiment 2
[0042] Example 2 Preparation of co-assembled multivalent epitope vaccine
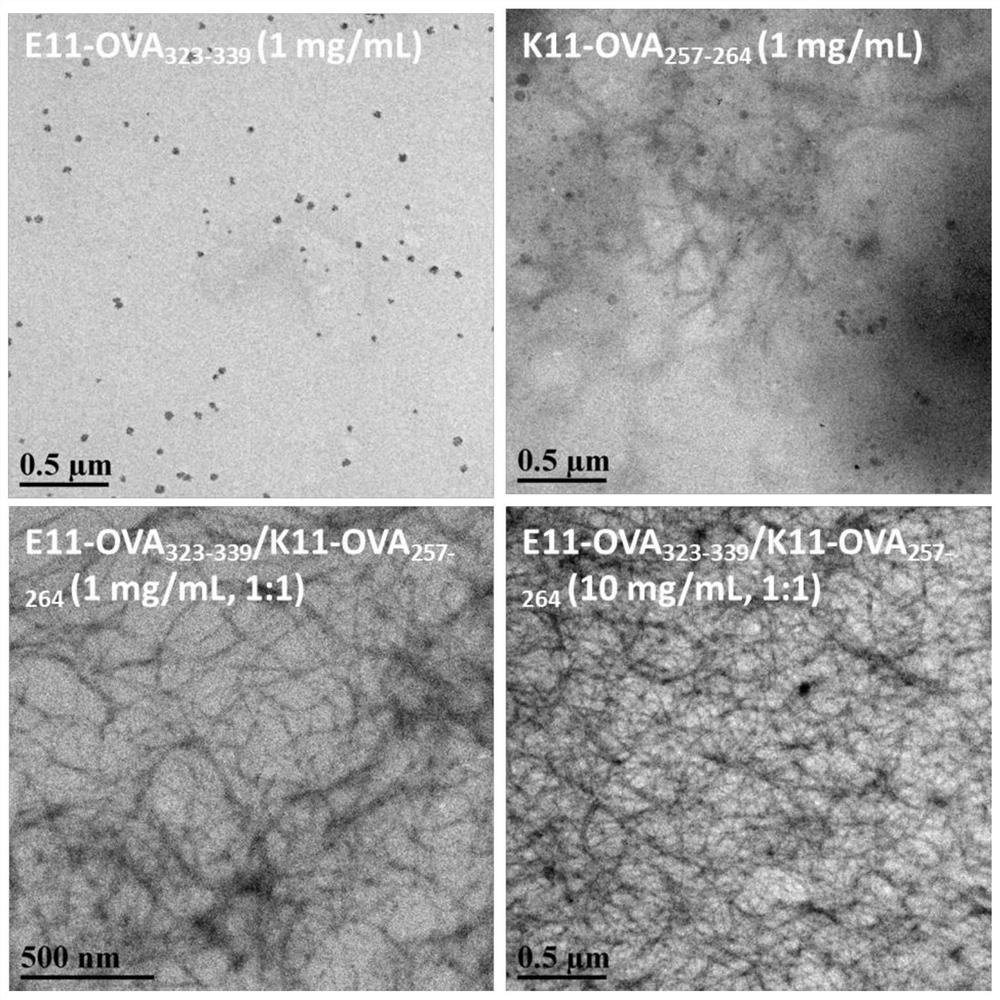
[0043] The small molecule polypeptides bound to different epitope peptides in Example 1 were dissolved in the aqueous solution at a molar ratio of 1:1, the concentration was adjusted, and nanostructures were formed through the co-assembly of the polypeptides to obtain a polymorphism in the form of hydrogel as a macroscopic expression. Epitope vaccines. For example: We bonded the KWKAKAKAKWK polypeptide with the SIINFEKL epitope, and then bonded the EWEAEAEAEAE polypeptide with the QAVHAAHAEINE epitope to construct two polypeptide sequences. The two polypeptides KWKAKAKAKWKGGGSIINFEK and EWEAEAEAEWEGGGQAVHAAHAEINE can be Spontaneous co-assembly occurs. The results show that as the concentration increases, the co-assembled structure changes from nanofibers to a mixture of three-dimensional porous structures and nanoparticles, and the macroscopic state changes from solution to semi-solid hydrogel.
Embodiment 3
[0044] Example 3: Study on in vitro activity of co-assembled epitope vaccines.
[0045] C57 / BL6 mouse bone marrow cells were isolated, and GM-CSF and IL-4 were added to the culture medium for 6 days, and immature dendritic cells (Dendritic cells, DCs) were harvested, and the solution prepared in Example 2 was The epitope vaccine was added to the medium to stimulate the differentiation of immature DC cells into mature DC cells, and after 24 hours of culture, flow cytometry (FACS) was used to detect the expression of the main surface markers MHCII, CD80, CD86 and CD40 of mature DC cells , using ELISA kits to detect the secretion of cytokines (IL-6, IL-12p40, IFN-γ and TNF-α). Image 6 As shown, the preliminary experimental results show that the epitope vaccine solution bonded to the polypeptide carrier can still maintain the activity of the epitope and effectively stimulate the maturation of DC cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com