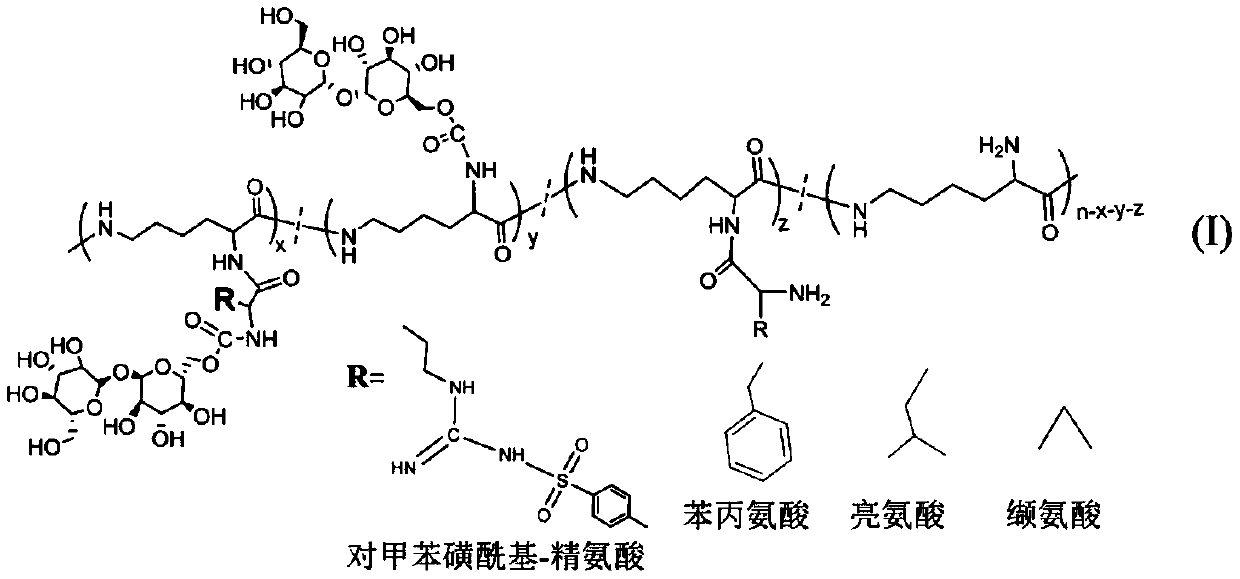
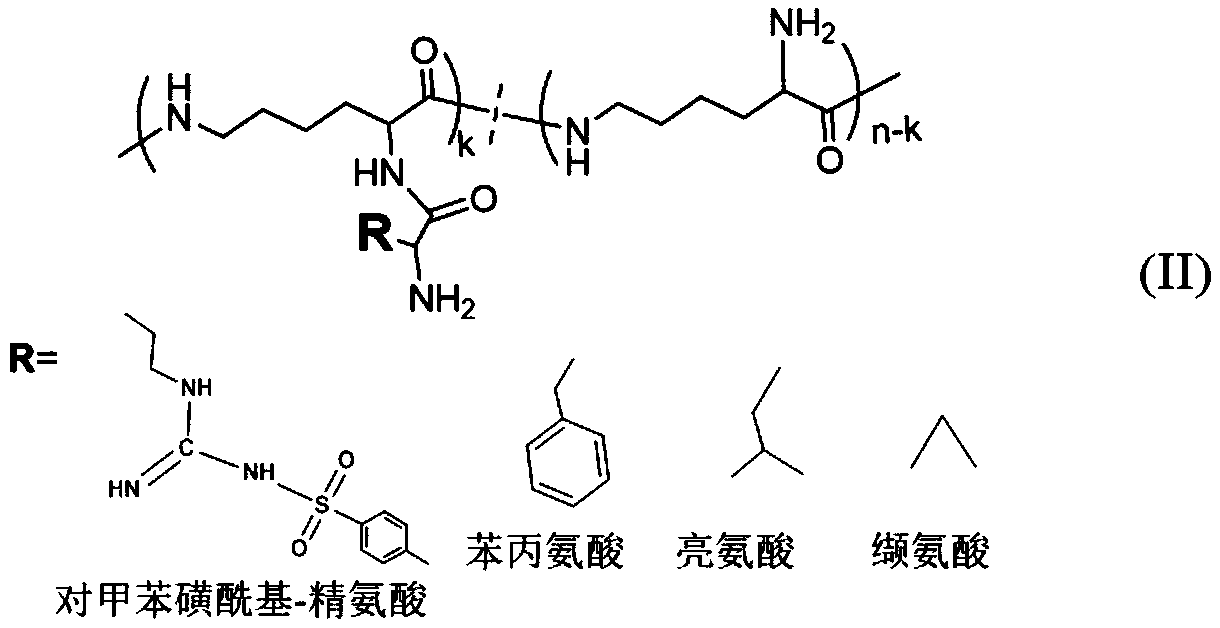
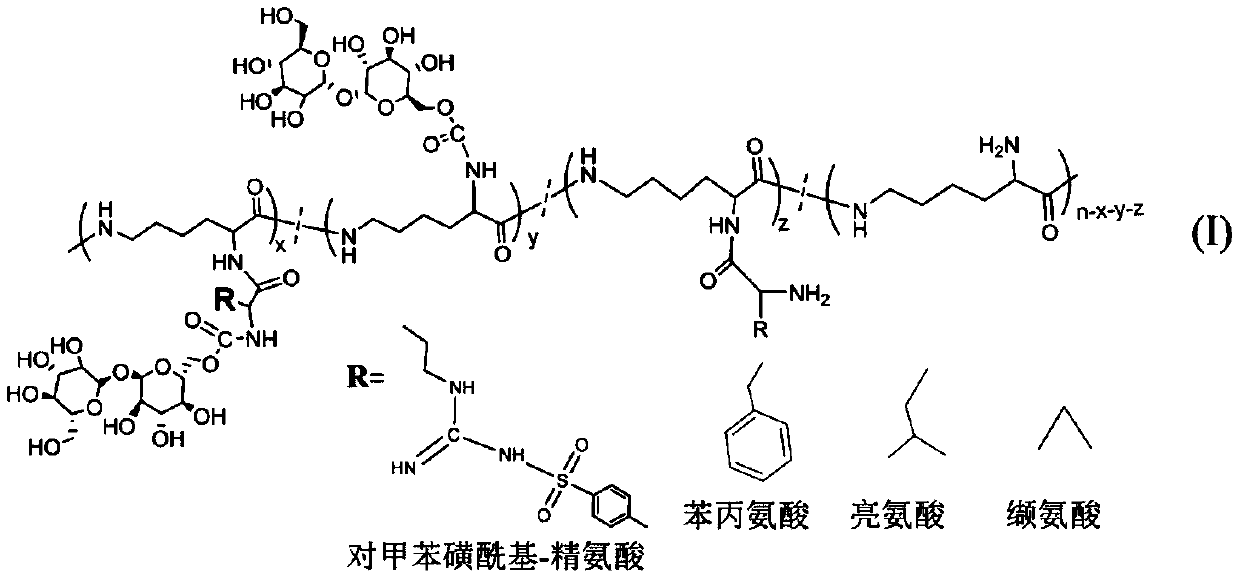
Glycopeptides of epsilon-polylysine-grafted-hydrophobic amino acid-grafted-trehalose and preparation method
A technology of hydrophobic amino acid and polylysine, which is applied in the field of biomedical materials, can solve the problems of non-membrane permeability, etc., and achieve the effect of easy operation, good biocompatibility, and easy availability of experimental raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Preparation of ε-polylysine-graft-p-toluenesulfonyl-arginine-graft-trehalose
[0026] 1) tert-butoxycarbonyl-p-toluenesulfonyl-arginine (1.07g, the amount of substance is 0.0025mol) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide 0.479 g, 0.0025mol) was dissolved in 11 mL of dimethyl sulfoxide to obtain a solution with a concentration of tert-butoxycarbonyl-p-toluenesulfonyl-arginine of 10 wt%, and stirred for 30 min;
[0027] 2) Add ε-polylysine (0.32g, the amount of amino substances is 0.0025mol) according to the molar ratio of ε-polylysine amino group to tert-butoxycarbonyl-p-toluenesulfonyl-arginine molar ratio of 1:1 The aqueous solution was reacted at room temperature for 72 hours, and after dialysis and freeze-drying, the product ε-polylysine-graft-tert-butoxycarbonyl-graft-p-toluenesulfonyl-arginine was obtained;
[0028] 3) The obtained ε-polylysine-graft-tert-butoxycarbonyl-graft-p-toluenesulfonyl-arginine product was fully dissolved by adding trifluoro...
Embodiment 2
[0038] (1) Preparation of ε-polylysine-graft-phenylalanine-graft-trehalose
[0039] 1) tert-butoxycarbonyl-phenylalanine (1.33g, the amount of substance is 0.005mol) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide 0.958g, 0.005mol ) was dissolved in 13 mL of dimethyl sulfoxide to obtain a solution with a concentration of tert-butoxycarbonyl-phenylalanine of 10 wt%, and stirred for 60 min;
[0040] 2) According to the amino group of ε-polylysine and tert-butoxycarbonyl-phenylalanine molar ratio 1:0.5, add ε-polylysine (0.32g, the amount of amino substance is 0.0025mol) aqueous solution and react at room temperature After 48 hours, after dialysis and freeze-drying, the product ε-polylysine-graft-phenylalanine-arginine was obtained;
[0041] 3) The obtained ε-polylysine-graft-phenylalanine-arginine product was fully dissolved by adding trifluoroacetic acid at a mass volume fraction of 15%, and stirred at room temperature for 2 hours;
[0042] 4) Precipitate with ether, collec...
Embodiment 3
[0051] (1) Preparation of ε-polylysine-graft-leucine-graft-trehalose
[0052] 1) tert-butoxycarbonyl-leucine (1.16g, the amount of substance is 0.005mol) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide 0.479g, 0.0025mol) Dissolve in 6 mL of dimethyl sulfoxide to obtain a solution with a concentration of tert-butoxycarbonyl-leucine of 20 wt%, and stir for 45 min;
[0053] 2) According to the amino group of ε-polylysine and tert-butoxycarbonyl-leucine molar ratio 1:0.75, add ε-polylysine (0.48g, the amount of amino substance is 0.00375mol) aqueous solution and react at room temperature for 56h , after dialysis and freeze-drying, the product ε-polylysine-graft-tert-butoxycarbonyl-leucine is obtained;
[0054] 3) The obtained ε-polylysine-graft-tert-butoxycarbonyl-leucine product was fully dissolved by adding trifluoroacetic acid at a mass volume fraction of 20%, and stirred at room temperature for 4 hours;
[0055] 4) Precipitate with ether, collect the precipitate, dissolve ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of grafting | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com