Electrochemical catalyst for oxygen evolution reaction, and preparation method and application thereof
An oxygen evolution reaction and catalyst technology, applied in the field of electrochemistry, can solve problems such as poor oxygen evolution performance, and achieve the effects of convenient operation, stable performance and energy saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] This embodiment provides a preparation method of an electrochemical oxygen evolution reaction catalyst, which specifically includes the following steps:
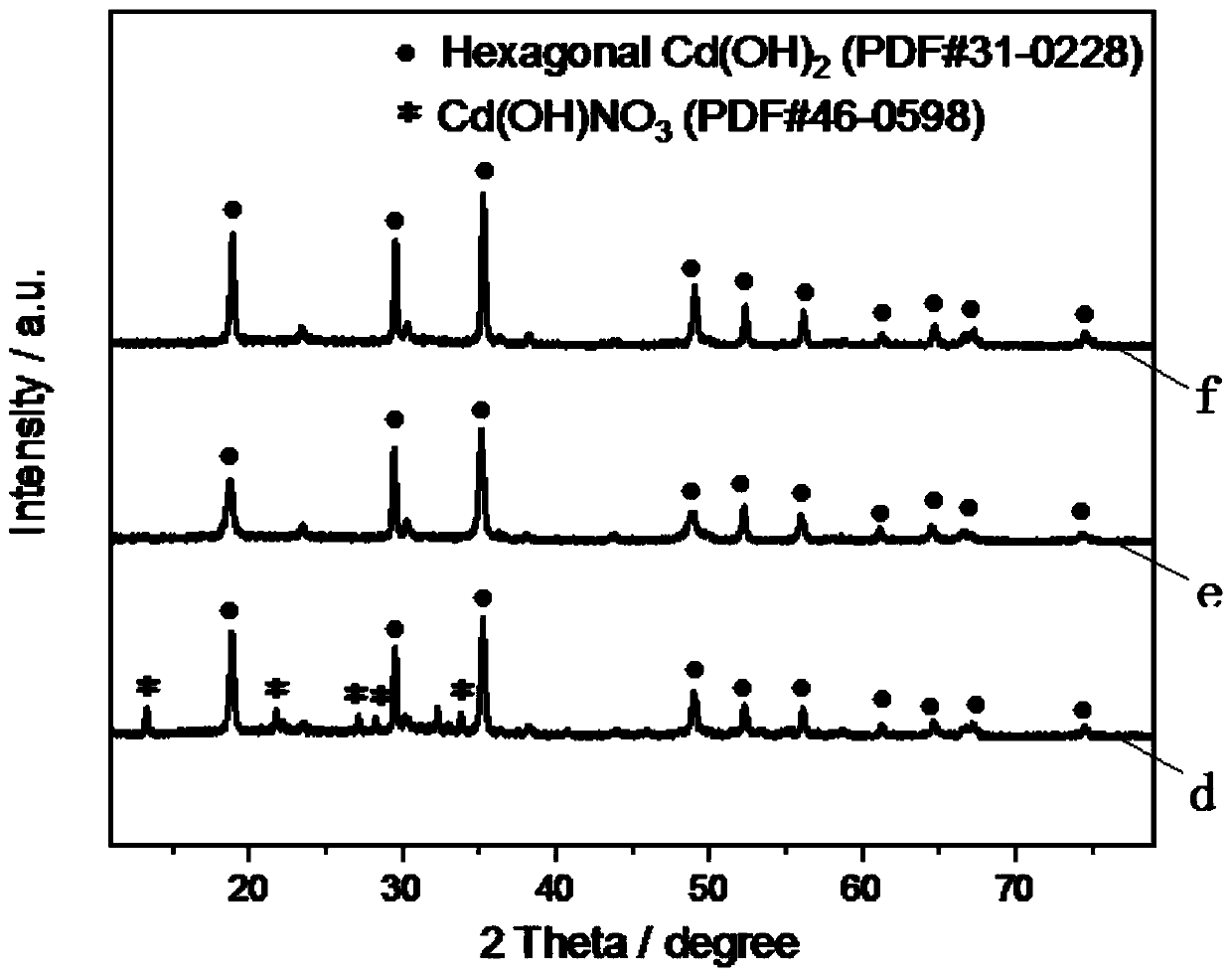
[0031] (1) in the 0.3mol / L cadmium nitrate aqueous solution of 10ml, dropwise add 10ml sodium hydroxide aqueous solution, the concentration of sodium hydroxide solution is 0.6mol / L, after fully reacting, reaction product is centrifugally filtered, and water washing filtrate, Dry the filtrate at a drying temperature of 60°C for 12 hours to obtain cadmium hydroxide;
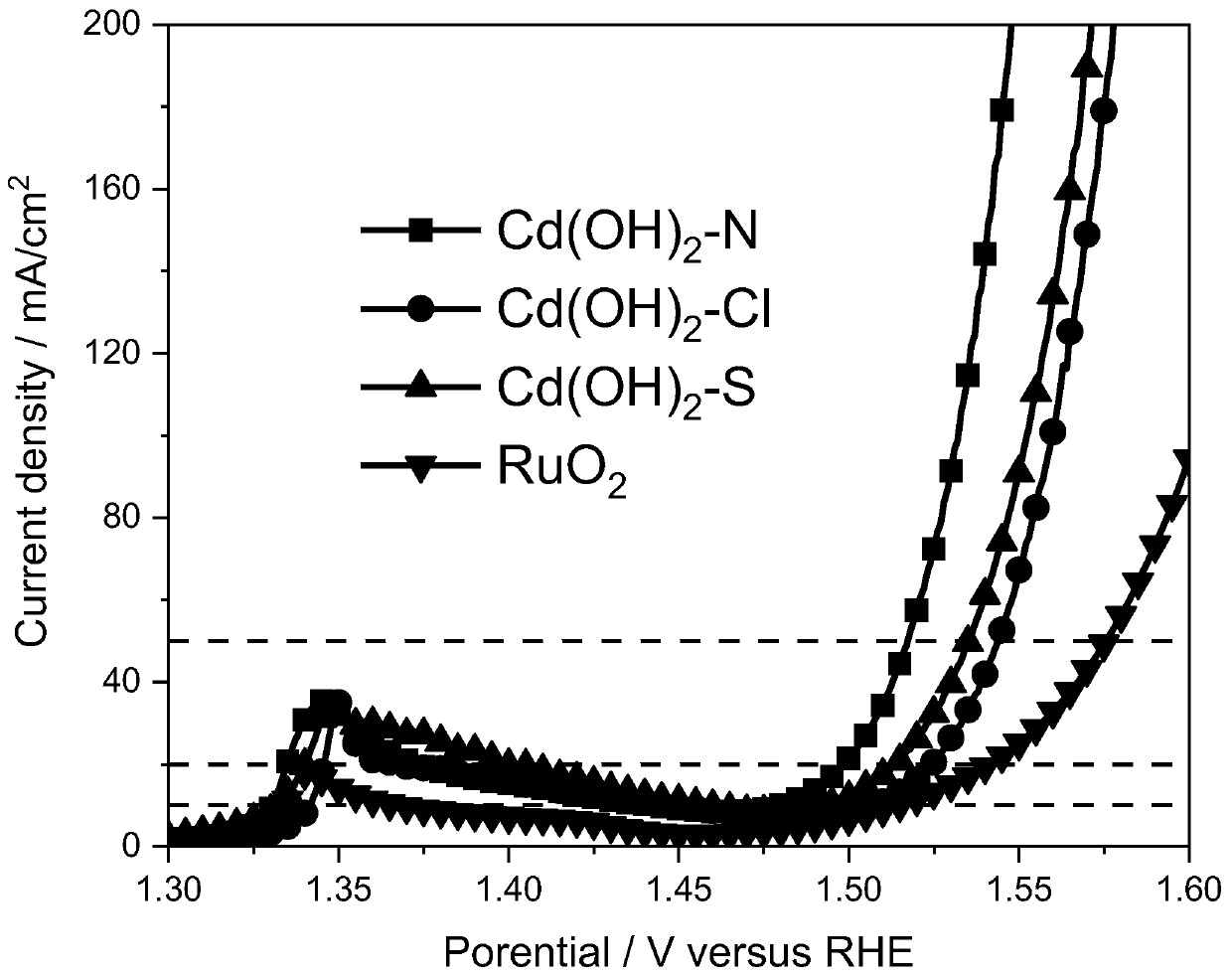
[0032] (2) Add 8 mg of cadmium hydroxide and 60 μl of Nafion solution to 2 ml of isopropanol, the concentration of Nafion solution is 5%, and ultrasonically mix for 1 hour to obtain a mixed solution. Take 10 μl of the mixed solution and apply it on the nickel foam , and the electrochemical oxygen evolution reaction catalyst was obtained after natural drying.
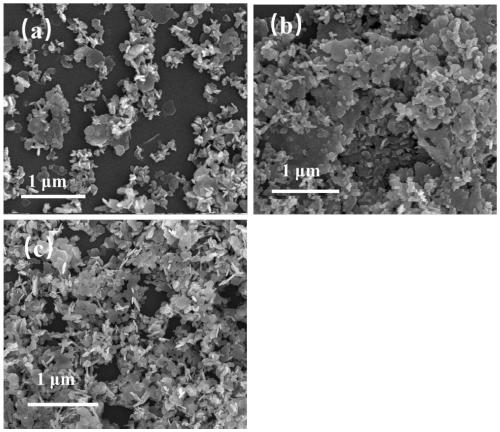
[0033] The SEM figure of cadmium hydroxide described in the present embodiment is as follows fi...
Embodiment 2
[0040] This embodiment provides a preparation method of an electrochemical oxygen evolution reaction catalyst, which specifically includes the following steps:
[0041] (1) Add 10ml of sodium hydroxide aqueous solution dropwise to the aqueous solution of 10ml of 0.3mol / L cadmium chloride, the concentration of sodium hydroxide solution is 0.6mol / L, after fully reacting, the reaction product is centrifugally filtered, washed with water and filtered matter, drying the filtrate at a drying temperature of 60°C for 12 hours to obtain cadmium hydroxide;
[0042] (2) Add 8 mg of cadmium hydroxide and 60 μl of Nafion solution to 2 ml of isopropanol, the concentration of Nafion solution is 5%, and ultrasonically mix for 1 hour to obtain a mixed solution. Take 10 μl of the mixed solution and apply it on the nickel foam , and naturally dried to obtain a cadmium hydroxide catalytic layer.
[0043] The SEM figure of the cadmium hydroxide described in the present embodiment is as follows f...
Embodiment 3
[0049] This embodiment provides a preparation method of an electrochemical oxygen evolution reaction catalyst, which specifically includes the following steps:
[0050] (1) In the aqueous solution of 0.3mol / L cadmium sulfate of 10ml, add 10ml sodium hydroxide aqueous solution dropwise, the concentration of sodium hydroxide solution is 0.6mol / L, after fully reacting, the reaction product is centrifuged and filtered, and the filter is washed with water , drying the filtrate at a drying temperature of 60°C for 12 hours to obtain cadmium hydroxide;
[0051] (2) Add 8 mg of cadmium hydroxide and 60 μl of Nafion solution to 2 ml of isopropanol, the concentration of Nafion solution is 5%, and ultrasonically mix for 1 hour to obtain a mixed solution. Take 10 μl of the mixed solution and apply it on the nickel foam , and naturally dried to obtain a cadmium hydroxide catalytic layer.
[0052] The SEM figure of the cadmium hydroxide described in the present embodiment is as follows fig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com