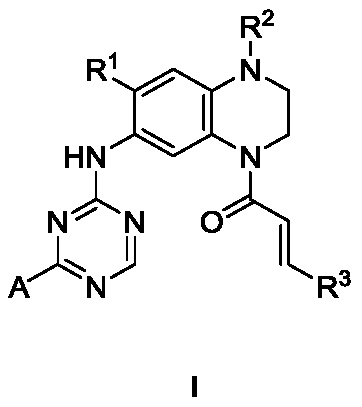
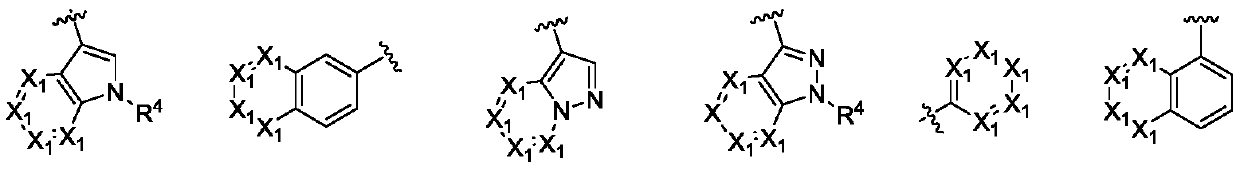
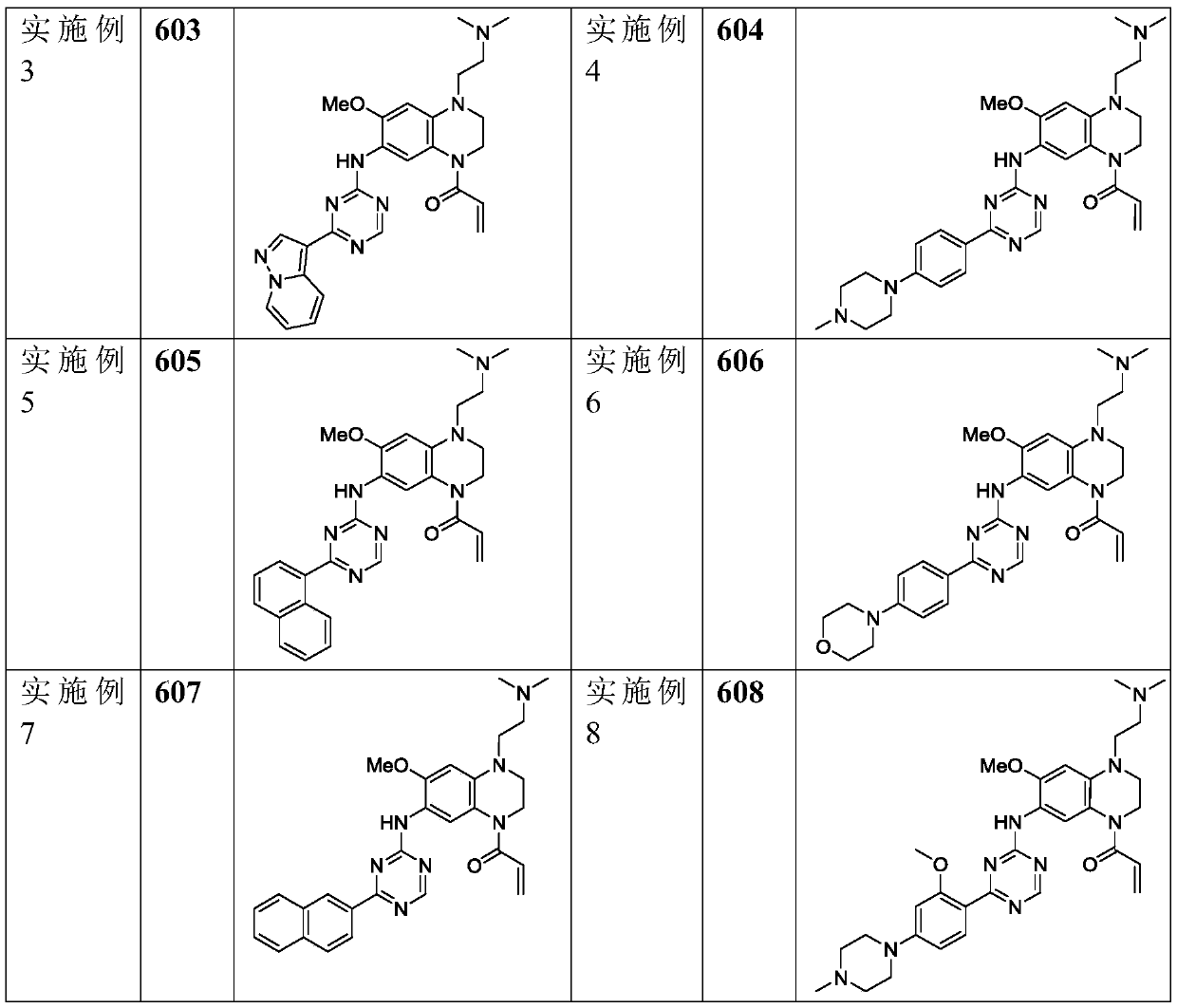
Triazine double aromatic ring derivative epidermal growth factor inhibitor and preparation method and application thereof
A technology of epidermal growth factor and aromatic ring, which is applied in the direction of active ingredients of heterocyclic compounds, drug combinations, and pharmaceutical formulas, and can solve problems such as cancer recurrence in patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: 2-{1'-N,N-dimethylaminoethyl-4'-acrylamido-7'-methoxy-1',2',3',4'-tetrahydro- 6'-quinoxaline}-4-(1"-methyl-1H-3"-indole) triazine (compound 601)
[0034]
[0035] Preparation of 2-chloro-4-(1'-methyl-1H-3'-indole)triazine:
[0036]
[0037] 2,4-dichlorotriazine (0.60g, 4mmol) was dissolved in ethylene glycol dimethyl ether (20mL), stirred under ice bath, ferric chloride (0.77g, 4.59mmol) was added in batches, and then The reaction was stirred at room temperature for 15 minutes. Then N-methylindole (0.68 g, 5.2 mmol) was added dropwise, followed by heating to 60° C. and stirring the reaction for 24 hours. Stop the reaction, lower to 0°C, add 3.5mL of methanol and 9mL of water, and then stir the reaction at room temperature for 3 hours. A large amount of solid precipitated, filtered, washed the filter cake with methanol, and dried to obtain 0.70 g of yellow solid, yield: 72%. LC / MS (ESI): m / z 245 (M+H) + .
[0038] Preparation of tert-butyl 4-fluoro-2...
Embodiment 2
[0062] Example 2: 2-{1'-N,N-dimethylaminoethyl-4'-acrylamido-7'-methoxy-1',2',3',4'-tetrahydro- Preparation of 6'-quinoxaline}-4-(1"-methyl-7"-aza-1H-3"-indole)triazine (compound 602):
[0063]
[0064] Preparation of 2-chloro-4-(1'-methyl-7'-aza-1H-3'-indole)triazine:
[0065]
[0066]1-methyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrrolo[2,3-B]pyridine ( 0.74g, 2.87mmol) and 2,4-dichlorotriazine (0.51g, 3.44mmol) were dissolved in ethylene glycol dimethyl ether (20mL), then dichlorodi-tert-butyl-(4-dimethyl Aminophenyl)phosphine palladium(II) (0.31g, 0.19mmol), 2M sodium carbonate solvent (3.2mL, 6.3mmol). Under nitrogen protection, stirred and heated to 80°C for 4 hours. The reaction was complete as detected by TLC. The reaction was stopped and diluted with water (1 mL). Ethyl acetate (25mL) extracted twice, anhydrous MgSO 4 After drying and concentration, the crude product was separated by flash column to obtain 457 mg of yellow solid, yield: 65%. ...
Embodiment 3
[0073] Example 3: 2-{1'-N,N-Dimethylaminoethyl-4'-acrylamido-7'-methoxy-1',2',3',4'-tetrahydro-6 '-quinoxaline}-4-(3"-pyrazol[1,5-a]pyridine)triazine (compound 603)
[0074]
[0075] Preparation of 2-chloro-4-(1'-methyl-1H-3'-indole)triazine:
[0076]
[0077] Pyrazolopyridine (1,5,-A)-3-boronate (0.70 g, 2.87 mmol) and 2,4-dichlorotriazine (0.51 g, 3.44 mmol) were dissolved in ethylene glycol dimethyl ether (20 mL), then dichlorodi-tert-butyl-(4-dimethylaminophenyl)phosphine palladium (II) (0.13 g, 0.19 mmol), 2M sodium carbonate solvent (32 mL, 6.3 mmol) was added with stirring. Under nitrogen protection, stir and heat to 80° C. for 4 hours. The reaction was complete as detected by TLC. The reaction was quenched and diluted with water (2 mL). Ethyl acetate (25mL) extracted twice, anhydrous MgSO 4 After drying and concentration, the crude product was separated by flash column to obtain 378 mg of yellow solid, yield: 57%. LC / MS (ESI): m / z 232 (M+H) + .
[0078] 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com