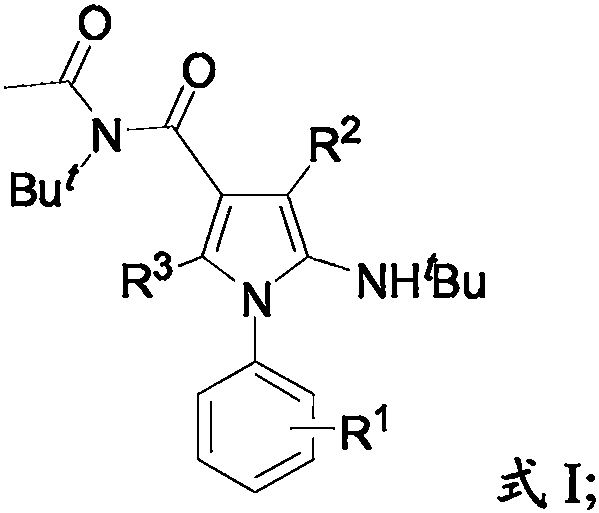
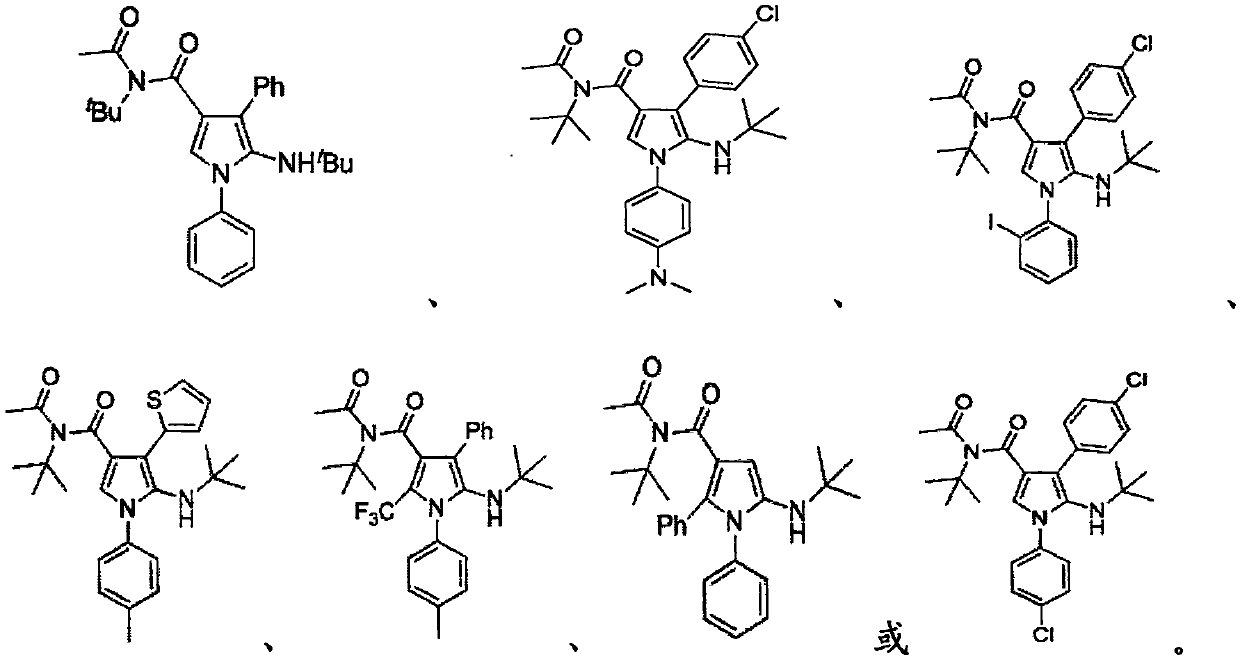
2-amino-4-acylpyrrole compound and production method thereof
An acylpyrrole and compound technology, which is applied in the field of 2-amino-4-acylpyrrole compounds and their preparation, can solve problems such as complicated synthesis of raw materials, and achieve the effects of simple method and wide applicability of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The present invention provides the preparation method of the 2-amino-4-acylpyrrole compound described in the above technical scheme, comprising the following steps:
[0035] Mixing arylamine, alkynyl ketone, tert-butyl isocyanide, acetate, catalyst and organic solvent for a synthesis reaction to obtain 2-amino-4-acylpyrrole compound;
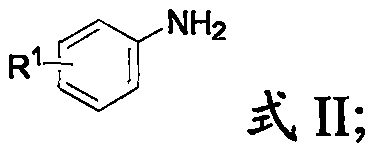
[0036] The arylamine has a structure shown in formula II;
[0037]
[0038] The alkynyl ketone has a structure shown in formula III:
[0039]
[0040] Among them, R 1 is -H, a halogen group, a methoxy group or a methyl group; R 2 is aryl, butyl or hydrogen; R 3 is aryl, trifluoromethyl or hydrogen.
[0041] In the present invention, the aromatic amine preferably includes aniline, N,N-dimethyl-4-aminoaniline, 2-iodoaniline, p-methylaniline, 4-chloroaniline, 4-methoxyaniline. In the present invention, the alkynyl ketone preferably includes phenylpropynyl formaldehyde, p-chlorophenylpropynyl formaldehyde, 2-thienylpropynyl formald...
Embodiment 1
[0050] Aniline (1a, 0.10M), phenylpropynylcarbaldehyde (2a, 0.12M), tert-butylisocyanate (0.3M), KOAc (1M) and palladium acetate (the molar percentage of palladium acetate and aniline is 5% ) was added into acetonitrile and mixed, the resulting reaction solution was heated to 80° C., and the synthesis reaction was carried out for 10 h, the resulting reaction solution was filtered, and the resulting filtrate was separated by column chromatography to obtain N-phenyl-2-amino-4-acylpyrrole ( 4a); the separation yield is 78%; the recorded concentration is the concentration of each raw material in the reaction solution; the synthetic reaction equation of embodiment 1 is as follows:
[0051]
[0052] 1 H NMR (500MHz, CDCl 3 )δ7.43-7.33 (m, 6H), 7.31 (d, J = 7.0Hz, 1H), 7.27 (d, J = 13.0Hz, 3H), 2.42 (s, 3H), 2.09 (s, 3H), 1.51(s, 9H), 0.55(s, 9H); 13 C NMR (126MHz, CDCl 3 )δ170.2, 169.2, 137.7, 136.6, 136.4, 134.6, 130.1, 129.7, 128.2, 127.6, 126.8, 125.6, 120.7, 120.3, 57.7, ...
Embodiment 2
[0054]N,N-dimethyl-4-aminoaniline (1b, 0.10M), p-chlorophenylpropynyl formaldehyde (2b, 0.12M), tert-butylisocyanate (0.3M), KOAc (1M) and palladium acetate (The molar percentage of palladium acetate and N, N-dimethyl-4-aminoaniline is 10%) was added to 1,2-dichloroethane and mixed, and the resulting reaction solution was heated to 80°C to carry out the synthesis reaction After 10h, the obtained reaction solution was filtered, and the obtained filtrate was separated by column chromatography to obtain N-(4-(N,N-dimethyl)amino)phenyl-2-amino-4-acylpyrrole (4b). The rate is 82%; the recorded concentration is the concentration of each raw material in the reaction solution; the synthetic reaction equation of embodiment 2 is as follows:
[0055]
[0056] 1 H NMR (500MHz, CDCl 3 )δ7-38-7.35(m, 2H), 7.33-7.30(m, 2H), 7.26(d, J=7.0Hz, 2H), 7.22(s, 1H), 6.76(d, J=9.0Hz, 2H ), 3.02(s, 6H), 2.07(s, 3H), 1.50(s, 9H), 0.59(s, 9H); 13 C NMR (126MHz, CDCl 3 C 29 h 37 ClN 4 o 2 508...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com