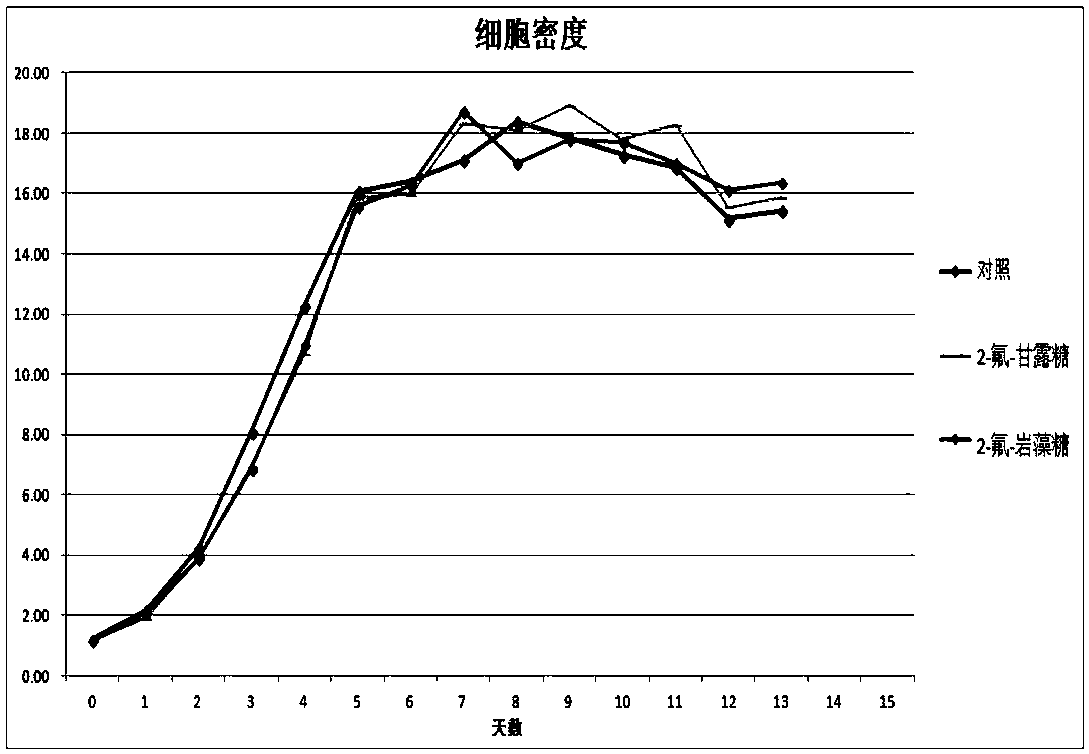
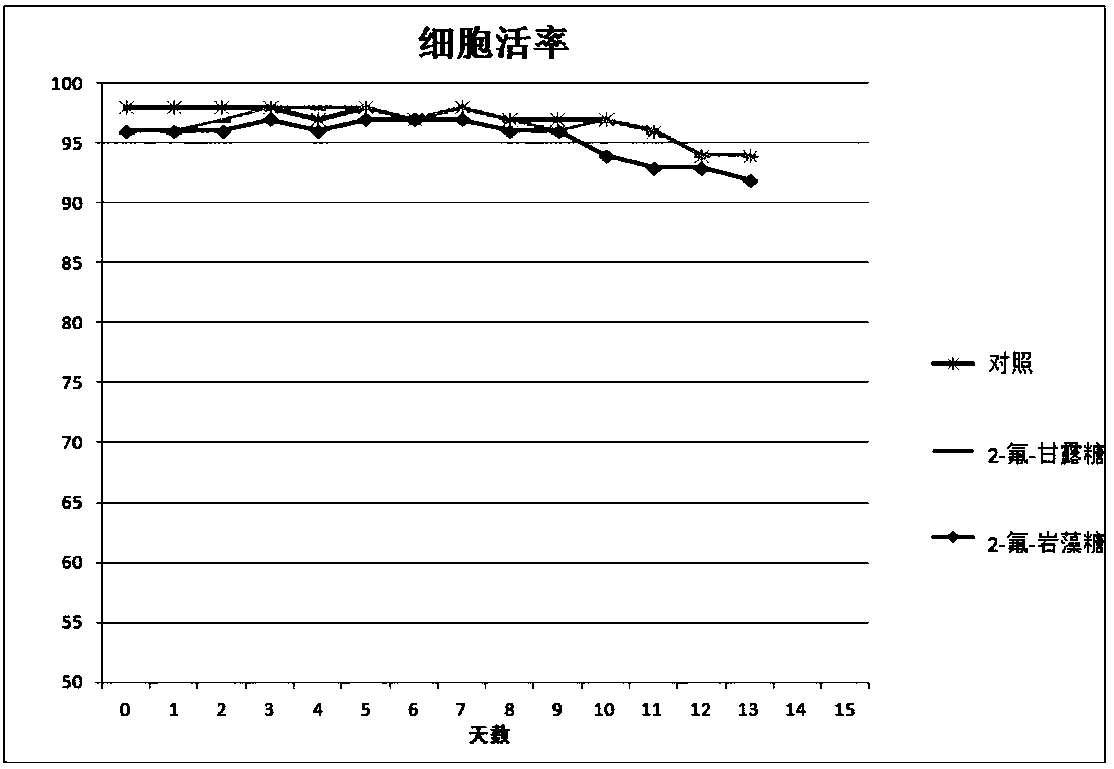
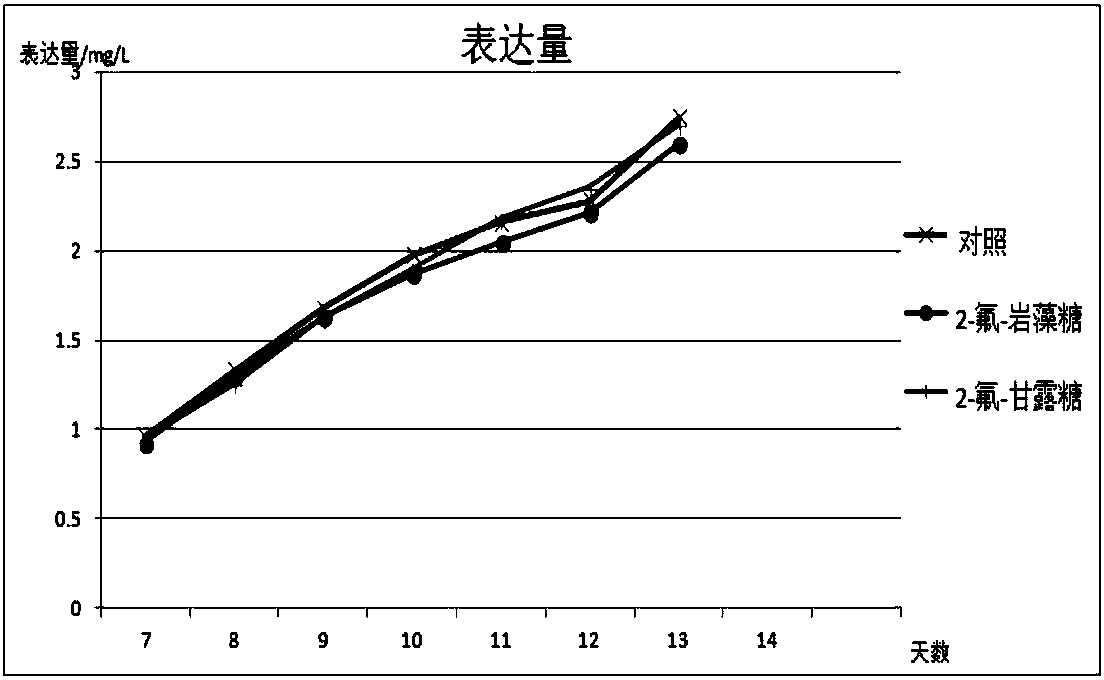
Antibody core fucosylation reducing method and composition
A technology of fucosylation and antibodies, applied in the field of a method and compounds used, can solve the problems of reducing core fucosylation, improving immunogenicity, and high price of raw material fucose, so as to reduce core rock Effects of glycosylation and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: Synthesis of 2-fluoro-mannose analogs, namely 2-deoxy-2-fluoro-1,3,4,6-tetra-oxo-acetyl-D-mannose
[0067]
Embodiment 11
[0069] Under nitrogen protection, D-mannose (50 g, 0.278 mol) was added into pyridine (500 mL), stirred and dissolved. Cool to 0°C, add acetic anhydride (500g, 4.09mol), heat up to 25°C and react for 16 hours. Concentrate under reduced pressure to remove pyridine and acetic anhydride, dissolve the residue with ethyl acetate (500mL), and successively wash with 1mol / L dilute hydrochloric acid (500mL*2), saturated sodium bicarbonate solution (500mL) and saturated sodium chloride solution (500mL) After washing, the organic phase was concentrated under reduced pressure to obtain 97.5 g of off-white solid, with a yield of 90%.
Embodiment 12
[0071] Under nitrogen protection, the compound prepared in Example 1.1 (97.5 g, 0.25 mol) was added into dichloromethane (1500 mL), stirred and dissolved, and cooled to 0°C. 40% hydrobromic acetic acid solution (400 mL) was slowly added, and the temperature was raised to 25°C to react for 3 hours. Washed successively with ice water (1500mL*2), saturated sodium bicarbonate solution (1500mL) and saturated sodium chloride solution (1500mL), the organic phase was concentrated under reduced pressure to obtain 82g of yellow oil with a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com