Metal organic framework material with petal-shaped core-shell structure and preparation method and application thereof
A metal-organic framework and core-shell structure technology, applied in chemical instruments and methods, other chemical processes, process efficiency improvement, etc., can solve the problems of slow kinetic behavior and low rare earth adsorption capacity, and achieve large adsorption capacity and preparation Convenient and widely applicable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
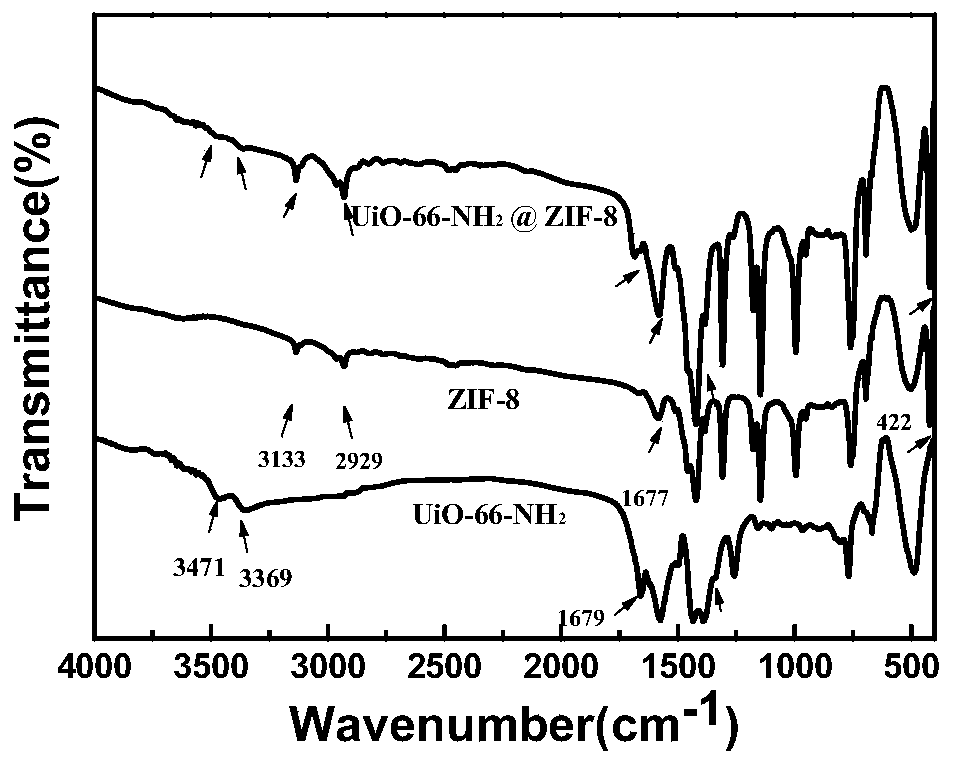
Image
Examples
Embodiment 1
[0031] Example 1 Metal-organic framework material UiO-66-NH with petal-shaped core-shell structure 2 Preparation of @ZIF-8
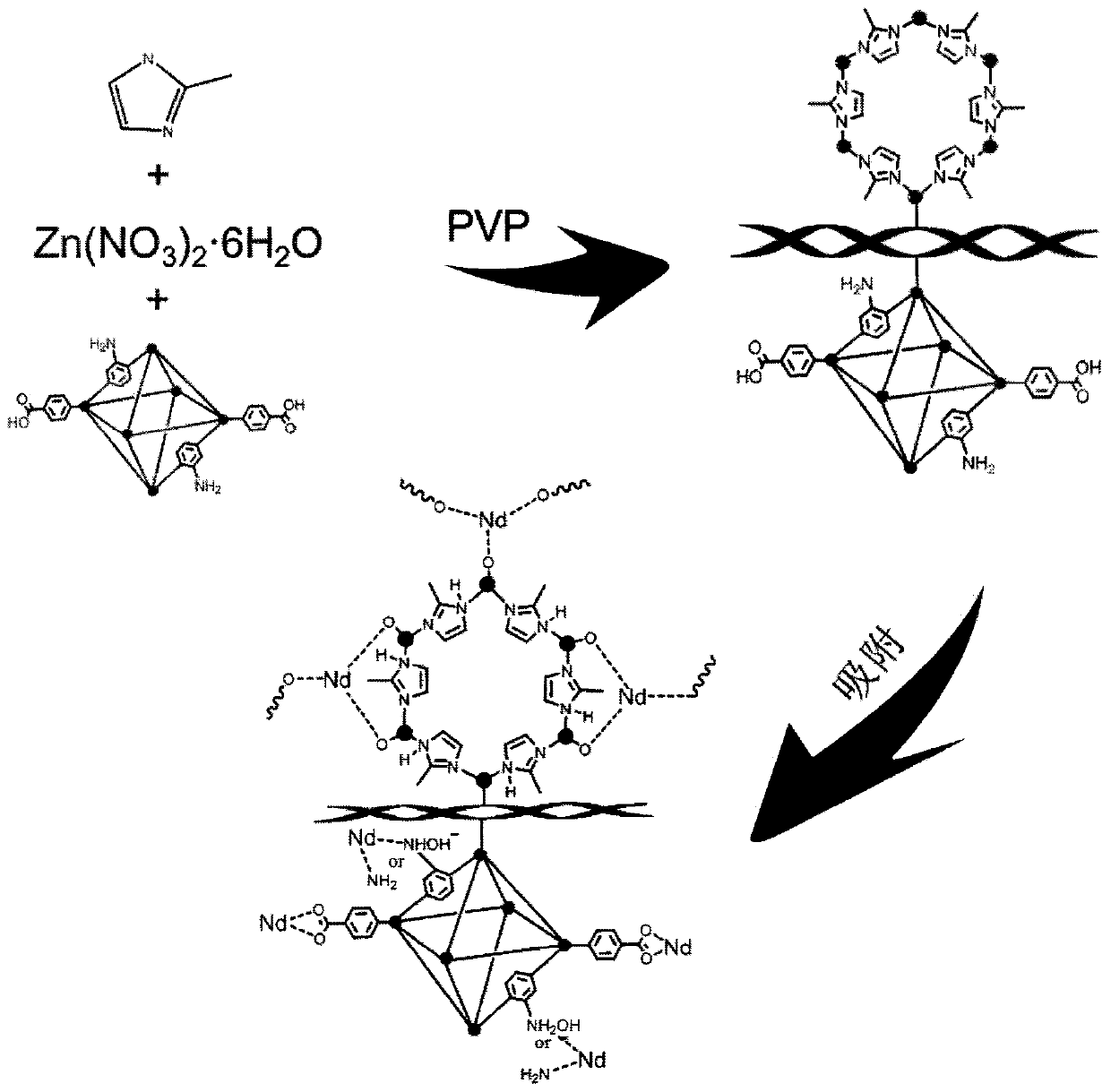
[0032] (1) Metal organic framework material UiO-66-NH with petal-like core-shell structure 2 Preparation of @ZIF-8: The synthesis process is as follows figure 1 as shown,
[0033] 1) Take 0.0745g of zirconium tetrachloride, 0.072g of 2-aminoterephthalic acid, 5.5mL of glacial acetic acid and 50mL of N,N-dimethylformamide and stir at room temperature. ℃ for 24h, cooled to room temperature, and the obtained UiO-66-NH after washing 2 .
[0034] 2) Weigh 10mg UiO-66-NH 2 In a round bottom beaker, ultrasonically disperse in 19ml of anhydrous methanol for 1h, then add 20mg of PVP and stir for 12h, then add 0.2g of zinc nitrate hexahydrate and stir for 12h, then add 0.25g of 2-methylimidazole, stir After 45min, centrifuge and dry to obtain the target product UiO-66-NH adsorbent with petal-shaped core-shell structure 2 @ZIF-8.
[0035] (2) Metal-organic fr...
Embodiment 2
[0039] Example 2 UiO-66-NH 2 Adsorption effect of @ZIF-8 on Nd(III), Eu(III), Gd(III), Er(III) at different acidity
[0040] 1. Method: Weigh 10mg UiO-66-NH respectively 2 @ZIF-8, add 10mL respectively to a concentration of 30mg L -1 Nd(III), Eu(III), Gd(III), Er(III) solutions, the pH of the solutions are 2, 3, 4, 5, 6 respectively, shake in a shaking box at 30°C and 180r / min for 24h . UiO-66-NH 2 The process of @ZIF-8 adsorbing Nd(III) is as follows figure 1 As shown, the adsorption results are as Figure 2d .
[0041] 2. by Figure 2d It can be seen that with the increase of pH value, UiO-66-NH 2 The adsorption rate of @ZIF-8 to rare earth ions gradually increases, and at pH=5, the adsorption rate of rare earths is over 90%, so as to achieve the rare earth elements Nd(III), Eu(III), Gd(III), Recovery of Er(III).
Embodiment 3
[0042] Example 3 UiO-66-NH 2 Adsorption isotherms of Nd(III), Eu(III), Gd(III), Er(III) on @ZIF-8 adsorbent
[0043] 1. Method: prepare the concentration of 20mg·g respectively -1 , 30mg·g -1 , 50mg·g -1 , 80mg·g -1 , 100mg·g -1 , 150mg·g -1 , 200mg·g -1 , 300mg·g -1 , 500mg·g -1 Rare earth ion solution, adjust the pH to 5. Weigh 10mgUiO-66-NH respectively 2 @ZIF-8, adding 10mL concentration is 20~500mg L -1 Nd(III), Eu(III), Gd(III), Er(III) solutions, the pH of the solutions are all 5, shake in a shaking box at 30°C and 180r / min for 24h. The result is as image 3 .
[0044] 2. by image 3 It can be seen that UiO-66-NH 2 The maximum adsorption capacities of @ZIF-8 for rare earth ions for Nd(III), Eu(III), Gd(III) and Er(III) are 249.90, 295.28, 316.22 and 340.95 mg g, respectively -1 . The maximum adsorption capacity of the adsorbent for rare earth follows the order of Nd(III)2 @ZIF-8 can efficiently recover rare earth ions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com