Light/temperature double sensitive hydrogel and preparation method thereof
A hydrogel and low-temperature technology, applied in the field of medical materials, can solve problems such as weak mechanical properties and poor stability, and achieve the effects of improving mechanical strength, shortening gelation time, and good development and application potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Prepare HBC with reference to patent CN201110214776.X;
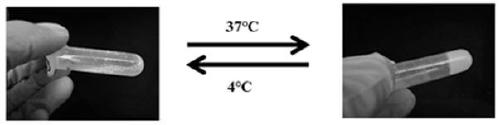
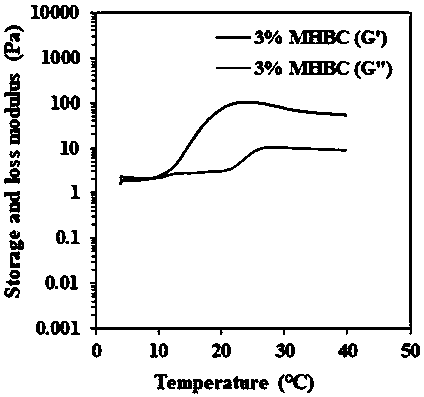
[0024] (2) Accurately weigh 0.5 g of HBC, dissolve it in 25 mL of deionized water, adjust the pH of the solution to 9.0 with 1M NaOH; slowly add 0.141 mL of glycidyl methacrylate (GMA) dropwise at a molar ratio of HBC:GMA=1:0.5 , stirred and reacted for 24 hours at low temperature and protected from light, and then adjusted the pH of the solution to neutral with 1M HCl; dialyzed the solution for 48 hours and freeze-dried to obtain MHBC; dissolved MHBC in deionized water at 4°C to a final concentration of 3 %, warming up to 25°C to obtain MHBC hydrogel;
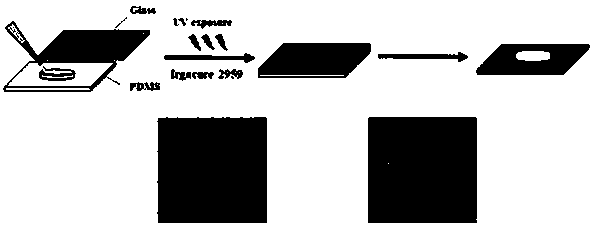
[0025] (3) Accurately weigh 30 mg of MHBC and dissolve it in 1 mL of deionized water containing 0.15 w / v photoinitiator Irgacure 2959, and irradiate the solution with ultraviolet light for 3 min to obtain photocrosslinked MHBC hydrogel.
Embodiment 2
[0027] (1) Prepare HBC with reference to patent CN201110214776.X;
[0028] (2) Accurately weigh 0.5 g of HBC, dissolve it in 25 mL of deionized water, adjust the pH of the solution to 9.0 with 1M NaOH; slowly add 0.212 mL of glycidyl methacrylate (GMA) dropwise at a molar ratio of HBC:GMA=1:0.75 , stirred and reacted for 36 hours at low temperature and protected from light, and then adjusted the pH of the solution to neutral with 1M HCl; dialyzed the solution for 48 hours and freeze-dried to obtain MHBC; dissolved MHBC in deionized water at 4°C to a final concentration of 3 %, warming up to 20°C to obtain MHBC hydrogel;
[0029] (3) Accurately weigh 30 mg of MHBC and dissolve in 1 mL of deionized water containing 0.20 w / v photoinitiator Irgacure 2959, and irradiate the solution with ultraviolet light for 2 min to obtain photocrosslinked MHBC hydrogel.
Embodiment 3
[0031] (1) Prepare HBC with reference to patent CN201110214776.X;
[0032] (2) Accurately weigh 0.5 g of HBC, dissolve it in 25 mL of deionized water, adjust the pH of the solution to 9.0 with 1M NaOH; slowly add 0.282 mL of glycidyl methacrylate (GMA) dropwise at a molar ratio of HBC:GMA=1:1 , stirred and reacted for 48 hours at low temperature and protected from light, and then adjusted the pH of the solution to neutral with 1M HCl; dialyzed the solution for 48 hours and freeze-dried to obtain MHBC; dissolved MHBC in deionized water at 4°C to a final concentration of 3 %, warming up to 18°C to obtain MHBC hydrogel;
[0033] (3) Accurately weigh 30 mg of MHBC and dissolve it in 1 mL of deionized water containing 0.25 w / v photoinitiator Irgacure 2959, and irradiate the solution with ultraviolet light for 1 min to obtain photocrosslinked MHBC hydrogel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com