Preparation method of injectable aquagel based on polyaspartic acid derivative
A technology of polyaspartic acid and derivatives, applied in the fields of biotechnology, biomedical materials and tissue engineering, can solve problems such as lack of stability in quality and molecular weight distribution, and achieve the effects of easy implementation, simple synthesis and low manufacturing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A method for preparing an injectable hydrogel based on polyaspartic acid derivatives, the steps are as follows:
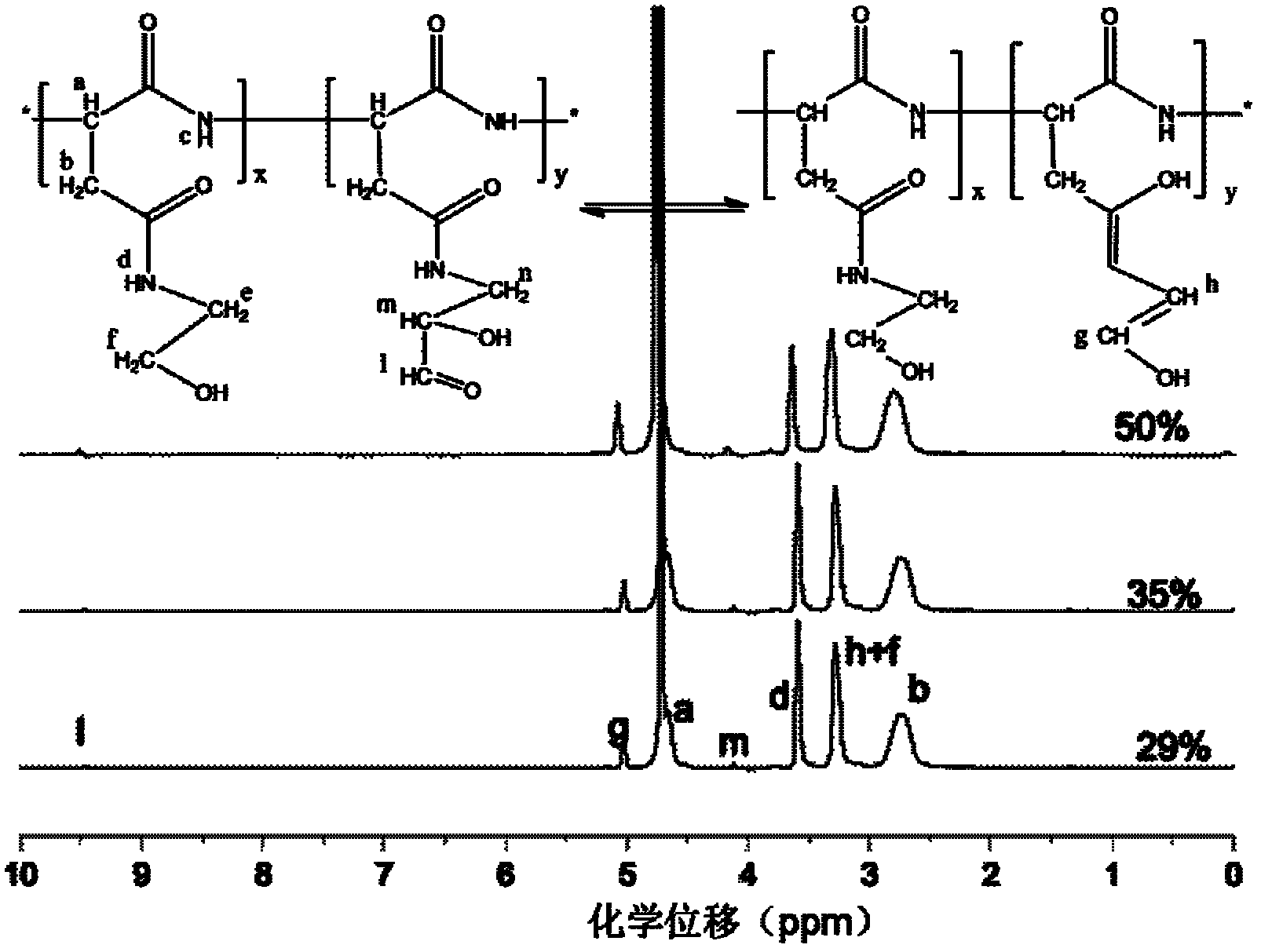
[0029] 1) Polysuccinimide is made by thermal polycondensation reaction:
[0030] Weigh 13.3g of L-aspartic acid powder, add 1mL of phosphoric acid with a concentration of 85% by weight in a 100mL three-neck flask, add sulfolane and 1,3,5-trimethylbenzene as a mixture in a ratio of 3:7 by volume Reaction solvent, a total of 30mL; under a nitrogen atmosphere, control the temperature at 180-220°C, stir mechanically for 4.5 hours, and remove the water and volatilized solvent generated during the reaction with a water separator; at the beginning of the reaction, L-Asp is a white crystal dispersed in the solvent , as the reaction temperature increases, a large amount of light yellow sticky lumps are generated, which is the intermediate polysuccinimide; after the reaction is completed, dissolve the product with DMF while it is hot, and add 4 mL of DMF per gram of t...
Embodiment 2
[0042] A method for preparing an injectable hydrogel based on polyaspartic acid derivatives, the steps are as follows:
[0043] 1) The preparation of polysuccinimide is the same as in Example 1.
[0044] 2) The preparation of polyaspartic acid hydrazide is the same as in Example 1.
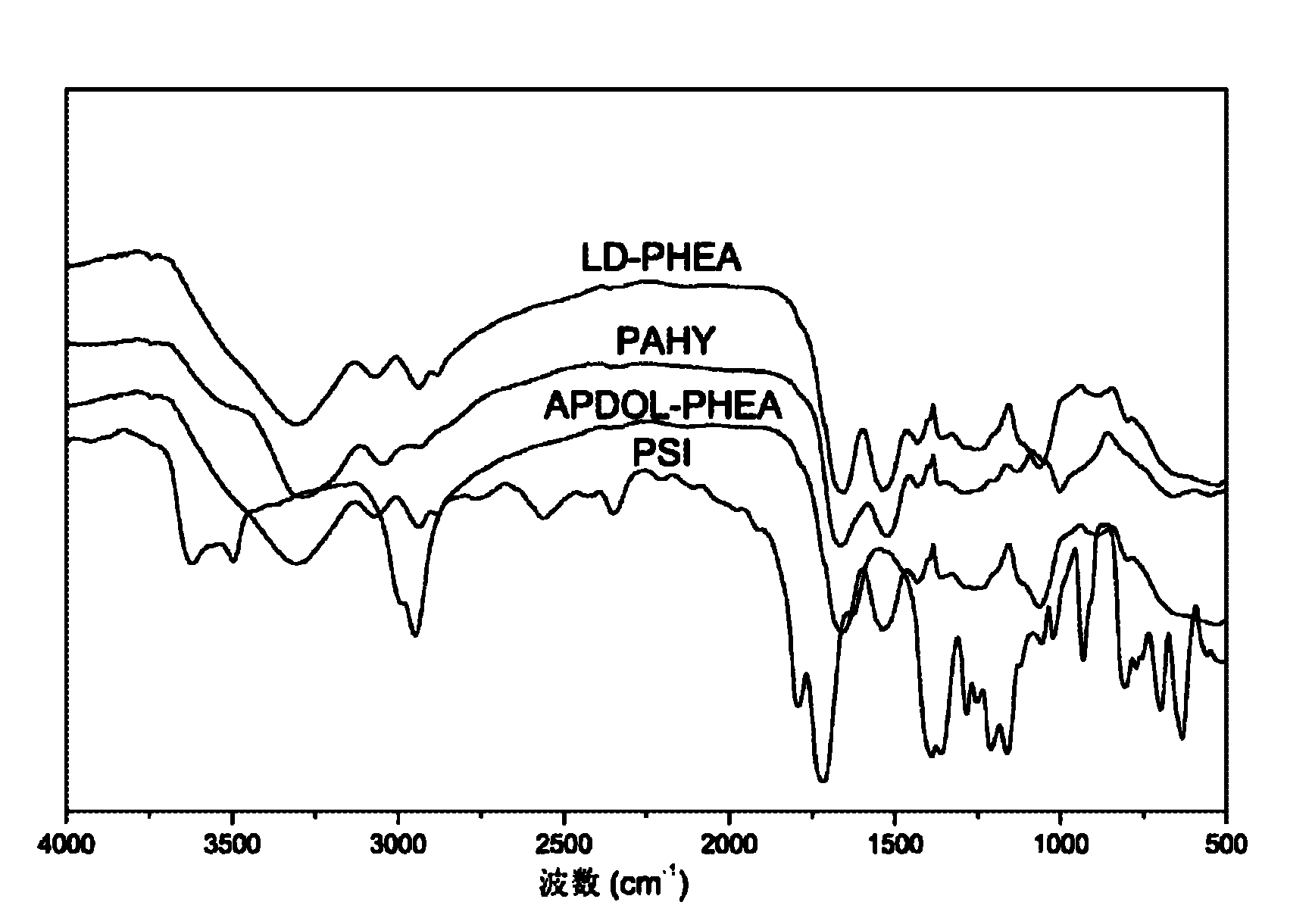
[0045] 3) Using 3-aminopropanediol and ethanolamine as nucleophiles, sequentially attacking polysuccinimide for ring-opening reaction, obtaining poly(2-hydroxyethylasparagine) modified by poly-3-amino-1,2-propanediol )(APDOL-PHEA):
[0046] Weigh 0.97g of vacuum-dried polysuccinimide, dissolve it in 10mL DMF, stir magnetically until it is completely dissolved to obtain a polysuccinimide solution; weigh 0.455g of 3-aminopropanediol and add it to 2.3mL DMF, Under the atmosphere of 3-aminopropanediol, the N,N-dimethylformamide solution was added dropwise to the polysuccinimide solution at a rate of 2-5 drops / min; reflux at 60°C for 8 hours, and the system After cooling to room temperature, add 0.7...
Embodiment 3
[0053] 1) The preparation of polysuccinimide is the same as in Example 1.
[0054] 2) The preparation of polyaspartic acid hydrazide is the same as in Example 1.
[0055] 3) Using 3-aminopropanediol and ethanolamine as nucleophiles, sequentially attacking polysuccinimide for ring-opening reaction, obtaining poly(2-hydroxyethylasparagine) modified by poly-3-amino-1,2-propanediol )(APDOL-PHEA):
[0056] Weigh 0.97g of vacuum-dried polysuccinimide, dissolve it in 10mL of N,N-dimethylformamide, and magnetically stir until completely dissolved to obtain a polysuccinimide solution; weigh 0.546g of 3-aminopropylene glycol to dissolve In 2.73mL DMF, under a nitrogen atmosphere, add the DMF solution of 3-aminopropanediol to the solution of polysuccinimide drop by drop at a rate of 2-5 drops / min; After cooling to room temperature, add 0.610 g of ethanolamine, and continue the reaction for 6 hours; after the reaction, remove 70% of DMF by rotary evaporation, pour 3-5 mL of the obtained...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com