Preparation and applications of 4-hydroxycoumarin compound
A kind of technology of hydroxycoumarin and compound, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
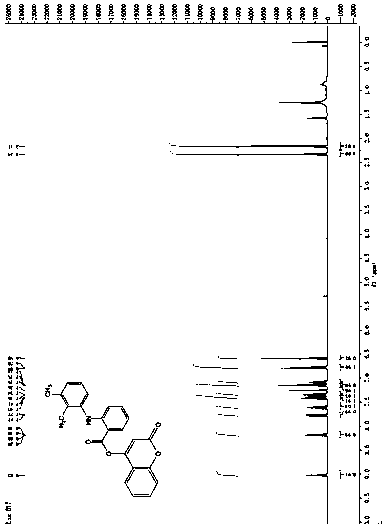
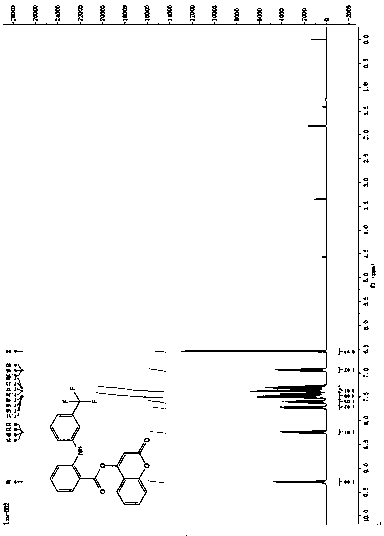
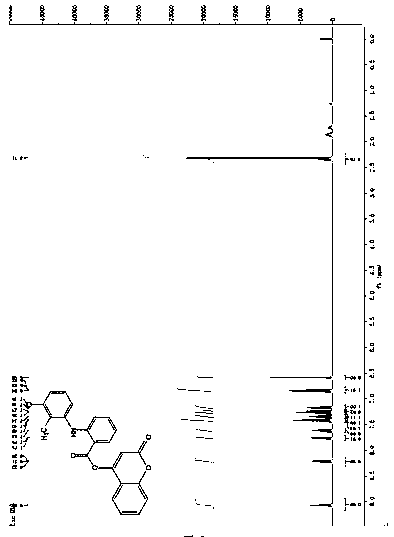
[0020] Preparation of the 4-hydroxycoumarin compounds: under nitrogen protection, 2.0 mmol 4-hydroxycoumarin, 2.0 mmol fenamic acid non-steroidal anti-inflammatory drugs (mefenamic acid, flufenamic acid and trofenamic acid) and 2.4 mmol 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDCI.HCl), slowly dropwise add 0.2 mmol dimethylaminopyridine solution as a catalyst, react at room temperature for 2 h, concentrate After purification with a silica gel column, the 4-hydroxycoumarin compounds were obtained with yields of 88%, 91% and 85%, respectively. refer to figure 1 , figure 2 with image 3 , compound (I): 1 H NMR (400 MHz, CDCl 3 ) δ 9.03 (s, 1H), 8.19 (dd, J =8.5, 1.5 Hz, 1H), 7.78 (dd, J = 7.9, 1.4 Hz, 1H), 7.65-7.58 (m, 1H), 7.44-7.36 (m, 2H), 7.35-7.29 (m, 1H), 7.15 (dd, J = 7.2, 4.9 Hz, 2H), 7.11-7.04(m, 1H), 6.79 (d, J = 8.4 Hz, 2H), 6.59 (s, 1H), 2.34 (s, 3H), 2.17 (s, 3H); HRMS (ESI) calcd for C 24 h 19 NO 4 [M + H] + : 385.4188, found ...
Embodiment 2
[0024] Evaluation of the inhibitory effect of 4-hydroxycoumarin compounds (I), (II) and (III) on lipopolysaccharide (LPS)-induced activation of microglial BV-2 by the determination of NO release (expressed as a percentage) , thus reflecting the in vitro anti-neuroinflammatory activity of the compound. The tested objects were all dissolved in DMSO to 10 mmol·L -1 , and then diluted with culture medium to the working concentration (20 μmol L -1 ),spare. BV-2 microglial cells in the logarithmic growth phase were taken to make cell suspension (primary isolation culture or BV-2 cell line), and 5×10 4 Cells were seeded in 96-well plates, 100 μL per well, and cultured overnight. Added LPS (100mg·L -1 ) and 20 μmol L -1 100 μL culture solution was added to the test target compound, the control group and the blank control group. 24 h after the addition, the release of NO in the cell solution of each well was detected by Griess reagent. The results show that the 4-hydroxycoumarin...
experiment example 3
[0032] Thirty healthy BALB / C female mice, weighing (30±4) g and aged (4±2) weeks, were randomly divided into 3 groups. Model group, drug group, and control group. The control group and the model group were intragastrically administered with distilled water (the volume was equivalent to that of the drug group, 0.3-0.5 mL / time), and the drug group was given 10 mg / (kg·d) for a total of 55 days. In addition to the control group, the model group and the drug group were established from the 8th day of intragastric administration, and the animals in each group were intraperitoneally injected with LPS 250 μg / (kg d), once every other day, for 7 consecutive days to establish LPS induction. Alzheimer's model. The blank group was injected intraperitoneally with normal saline (the volume is equivalent to that of other groups, 0.2 mL / time), once every other day, for 7 consecutive days.
[0033] 1 Detection of NO content
[0034] Decapitation was performed, and the brain was quickly removed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com