Composite metal oxide, positive electrode active material, positive electrode, sodium secondary battery, and method for producing composite metal oxide
A cathode active material, composite metal technology, applied in active material electrodes, secondary batteries, chemical instruments and methods, etc., can solve the problems of not being abundant in resources, depletion of lithium resources, etc., to achieve non-gelling, high water resistance, etc. , Improve the effect of coating
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0214] 153.7 mg of boric acid (H 3 BO 3 ) into an agate mortar, using sodium carbonate (Na 2 CO 3 ), manganese (IV) oxide (MnO 2 ), Iron(III) oxide (Fe 2 o 3 ) and nickel (II) oxide (NiO) as a metal-containing compound were weighed in a total of 15 g such that the molar ratio of Na:Mn:Fe:Ni was 0.99:0.35:0.30:0.35, put into an agate mortar, and then dried mixed to obtain a mixture.
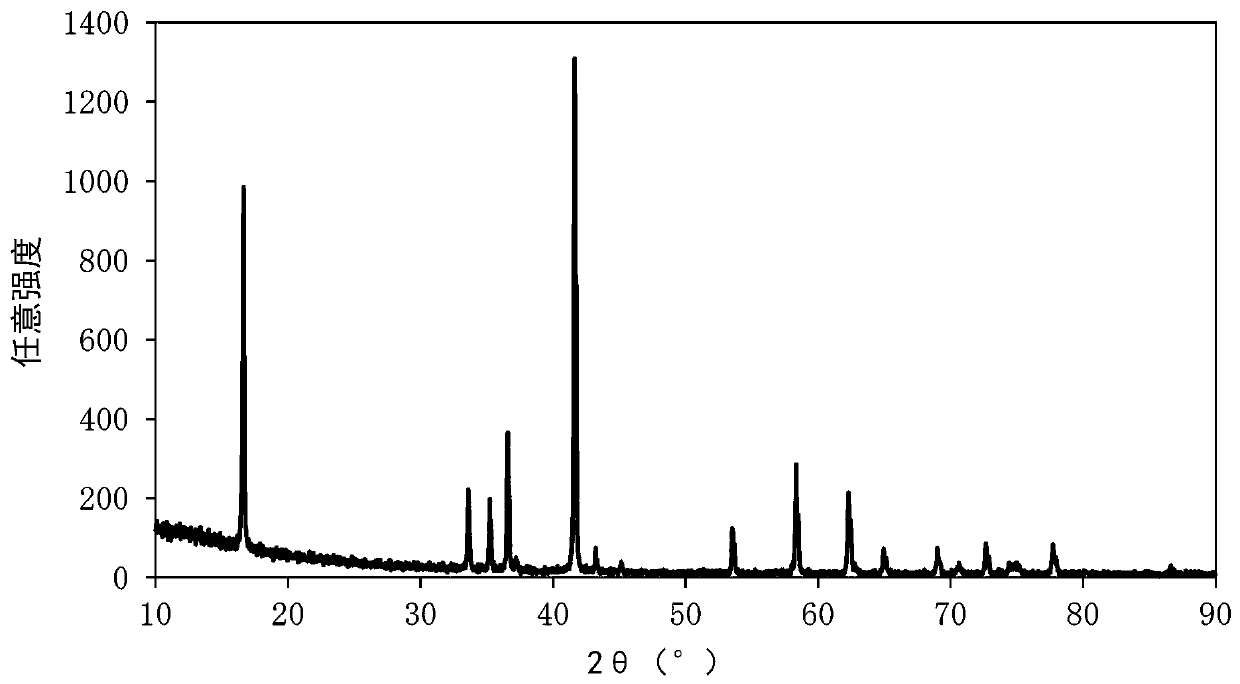
[0215] The obtained mixture was filled in an alumina crucible, heated in an air atmosphere using an electric furnace, and kept at 900° C. for 12 hours as a second firing step after holding at 650° C. for 4 hours as a first firing step. Then, the above mixture was fired and cooled to room temperature to obtain composite metal oxide 1 (Na 0.99 mn 0.35 Fe 0.30 Ni 0.35 o 2 ). As a result of powder X-ray diffraction measurement of composite metal oxide 1, the crystal structure of composite metal oxide 1 belongs to α-NaFeO 2 type crystal structure ( figure 2 ). The α-NaFeO 2 The peak ha...
Embodiment 2
[0217] 153.6 mg of boric acid (H 3 BO 3 ) into an agate mortar, using sodium carbonate (Na 2 CO 3 ), manganese (IV) oxide (MnO 2 ), Iron(III) oxide (Fe 2 o 3 ) and nickel (II) oxide (NiO) as a metal-containing compound were weighed in a total of 15 g so that the molar ratio of Na:Mn:Fe:Ni was 0.99:0.30:0.40:0.30, put into an agate mortar, and then dried mixed to obtain a mixture.
[0218] The obtained mixture was filled in an alumina crucible, heated in an air atmosphere using an electric furnace, and kept at 900° C. for 12 hours as a second firing step after holding at 650° C. for 4 hours as a first firing step. Then, the above mixture was fired and cooled to room temperature, thereby obtaining the composite metal oxide 2 (Na 0.99 mn 0.30 Fe 0.40 Ni 0.30 o 2 ). The result of powder X-ray diffraction measurement of composite metal oxide 2 is that the crystal structure of composite metal oxide 2 belongs to α-NaFeO 2 type crystal structure ( image 3 ). The α-NaFe...
Embodiment 3
[0220] 153.2 mg of boric acid (H 3 BO 3 ) into an agate mortar, using sodium carbonate (Na 2 CO 3 ), manganese (IV) oxide (MnO 2 ), Iron(III) oxide (Fe 2 o 3 ) and nickel (II) oxide (NiO) as a metal-containing compound were weighed in a total of 15 g such that the molar ratio of Na:Mn:Fe:Ni was 0.99:0.31:0.41:0.28, put into an agate mortar, and then dried mixed to obtain a mixture.
[0221] The obtained mixture was filled in an alumina crucible, heated in an air atmosphere using an electric furnace, and kept at 900° C. for 12 hours as a second firing step after holding at 650° C. for 4 hours as a first firing step. Then, the above mixture was fired and cooled to room temperature, thereby obtaining the composite metal oxide 3 (Na 0.99 mn 0.31 Fe 0.41 Ni 0.28 o 2 ). The result of powder X-ray diffraction measurement of composite metal oxide 3 is that the crystal structure of composite metal oxide 3 belongs to α-NaFeO 2 type crystal structure ( Figure 4 ). The α-N...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com