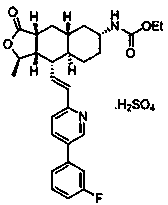
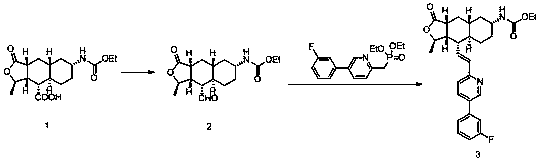
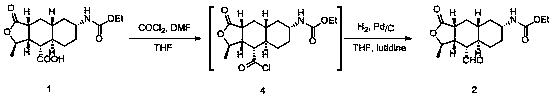
Synthesis method of vorapaxar sulfate intermediate aldehyde group compound
A synthesis method and technology of intermediates, applied in the field of medicinal chemical synthesis, can solve the problems of not using industrialized production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]Add 339 mg (0.001 mol) of Vorapasar sulfate intermediate carboxylic acid 1, 10 ml of tetrahydrofuran, and 164 mg (0.0012 mol) of isobutyl chloroformate to a three-necked flask equipped with a thermometer and magnetic stirring, and cool to 0 °C, then slowly added 310 mg (0.0024 mol) of diisopropylethylamine to the reaction system, and continued to stir for 10 min. Subsequently, 275 mg (0.0025 mol) of thiophenol was added to the reaction system, and after the addition was completed, the temperature was raised to 25 °C, and stirring was continued for 1 h. After the reaction was completed, 10 ml of dichloromethane was added to the reaction system, the reaction solution was washed with aqueous sodium bicarbonate solution, and the reaction solution was washed with aqueous sodium chloride solution, dried, and concentrated to obtain vorapaxer sulfate intermediate 7. Intermediate 7 was transferred to a three-necked flask with magnetic stirring, a thermometer, and a reflux condens...
Embodiment 2
[0051] Add 339 mg (0.001 mol) of Vorapasar sulfate intermediate carboxylic acid 1, 10 ml of tetrahydrofuran, and 164 mg (0.0012 mol) of isobutyl chloroformate to a three-necked flask equipped with a thermometer and magnetic stirring, and cool to 0 °C, then slowly added 310 mg (0.0024 mol) of diisopropylethylamine to the reaction system, and continued to stir for 10 min. Subsequently, 350 mg (0.0025 mol) of p-methoxythiophenol was added to the reaction system, and after the addition was completed, the temperature was raised to 25 °C, and stirring was continued for 1 h. After the reaction was completed, 10 ml of dichloromethane was added to the reaction system, the reaction solution was washed with aqueous sodium bicarbonate solution, and the reaction solution was washed with aqueous sodium chloride solution, dried, and concentrated to obtain vorapaxer sulfate intermediate 7. Intermediate 7 was transferred to a three-necked flask with magnetic stirring, a thermometer, and a refl...
Embodiment 3
[0054] Add 339 mg (0.001 mol) of Vorapasar sulfate intermediate carboxylic acid 1, 10 ml of tetrahydrofuran, and 164 mg (0.0012 mol) of isobutyl chloroformate to a three-necked flask equipped with a thermometer and magnetic stirring, and cool to 0 °C, then slowly added 310 mg (0.0024 mol) of diisopropylethylamine to the reaction system, and continued to stir for 10 min. Subsequently, 388 mg (0.0025 mol) of p-nitrothiophenol was added to the reaction system, and after the addition was completed, the temperature was raised to 25 °C, and stirring was continued for 1 h. After the reaction was completed, 10 ml of dichloromethane was added to the reaction system, the reaction solution was washed with aqueous sodium bicarbonate solution, and the reaction solution was washed with aqueous sodium chloride solution, dried, and concentrated to obtain vorapaxer sulfate intermediate 7. Intermediate 7 was transferred to a three-necked flask with magnetic stirring, a thermometer, and a reflux...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com