Indium complex taking APT as ligand and having potential leaving group as well as synthesis method and application of the indium complex
A kind of technology of leaving group and synthesis method, applied in the field of indium complex and its synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
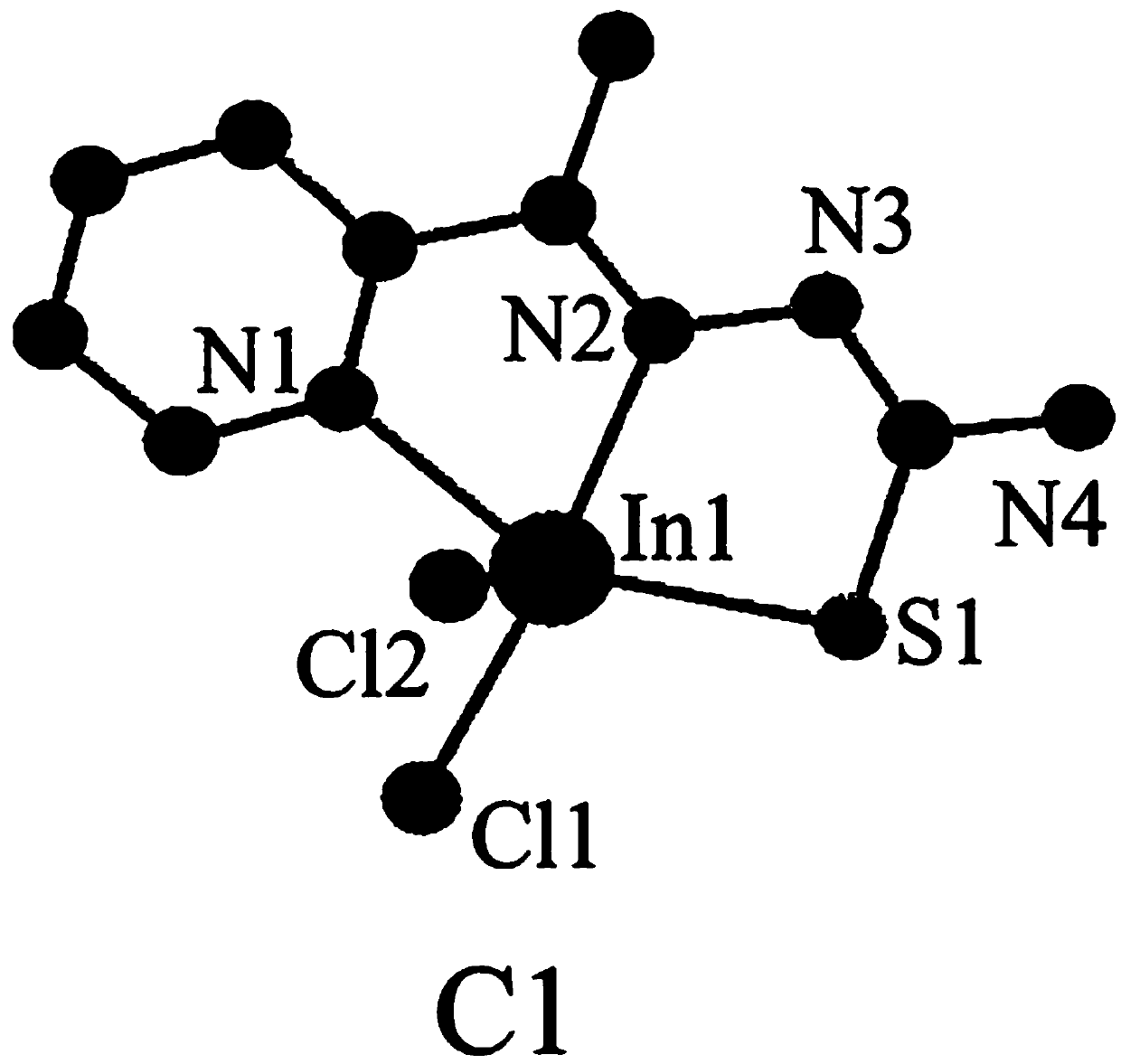
[0028] The synthetic method of C1 indium complex is:
[0029] 1) Dissolve 2-acetylpyridine (363mg, 3mmol) in a mixture of 10mL methanol and 10mL acetonitrile, add thiosemicarbazide (273mg, 3mmol), drop 3-4 drops (about 1mL) of acetic acid, and reflux at 85°C After 10h, the reaction was concentrated under reduced pressure, extracted with ethyl acetate, washed with saturated sodium bicarbonate and water successively, and the eluent was separated by silica gel column chromatography (the eluent was a mixture of petroleum ether and ethyl acetate, petroleum ether and acetic acid The volume ratio of ethyl ester is 10:1) to obtain ligand L1 (0.525g, 90.2%, pale yellow solid);
[0030] Yield: 0.525g, 90.2%, C 8 h 10 N 4 S: C, 49.46; H, 5.19; N, 28.84; S, 16.51. Found: C, 49.41; H, 5.18; N, 28.90; (s,NH),3147(m,aromatichydrogen),1600(m),1512(s),1450(s,aromatic),1398(m,C=N),1291(s,thioamide),1156(s) ,1101(s),882(m,C-H),714(m,C=S),596(m). 1 H NMR (400MHz, DMSO-d 6 )δ10.32(s,1H),8.5...
Embodiment 2
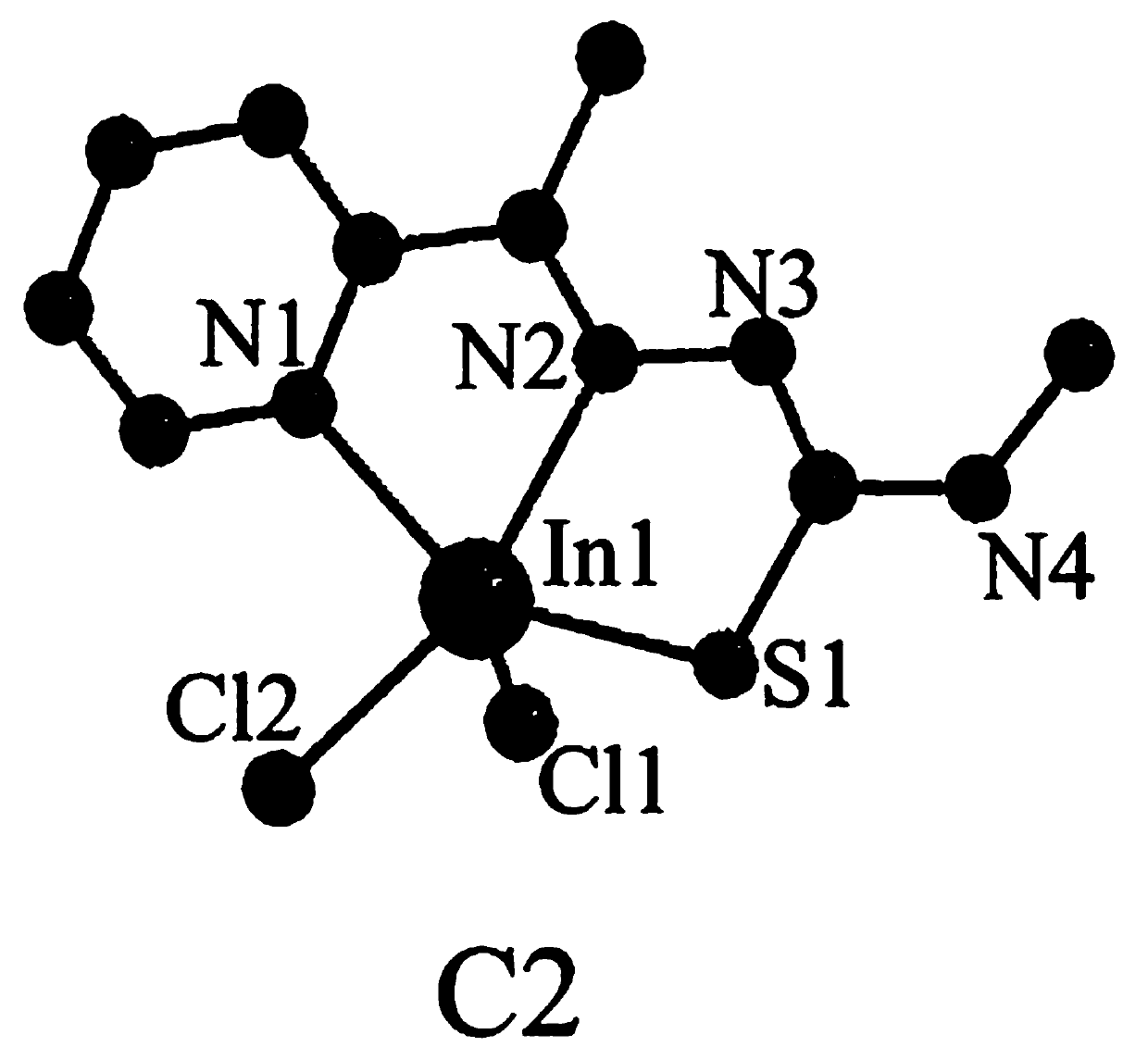
[0034] The synthetic method of C2 indium complex is:
[0035] 1) Dissolve 2-acetylpyridine (363mg, 3mmol) in a mixture of 10mL methanol and 10mL acetonitrile, add 4-methylthiosemicarbazide (315mg, 3mmol), add dropwise 3-4 drops (about 1mL) of acetic acid , refluxed at 85° C. for 10 h, concentrated under reduced pressure at the end of the reaction, extracted with ethyl acetate, washed with saturated sodium bicarbonate and water successively, and separated by silica gel column chromatography eluent (the eluent was a mixture of petroleum ether and ethyl acetate, petroleum The volume ratio of ether and ethyl acetate is 10:1), to obtain ligand L2 (0.520g, 83.3%, pale yellow solid);
[0036] Yield: 0.520g, 83.3%, C 8 h 10 N 4S: C, 51.90; H, 5.81; N, 26.90; S, 15.39. Found: C, 51.87; H, 5.80; N, 26.92; (s,NH),3143(m,aromatichydrogen),1599(m),1511(s),1450(s,aromatic),1388(m,C=N),1293(s,thioamide),1157(s) ,1101(s),883(m,C-H),715(m,C=S),594(m). 1 H NMR (400MHz, DMSO-d 6 )δ10.34(s...
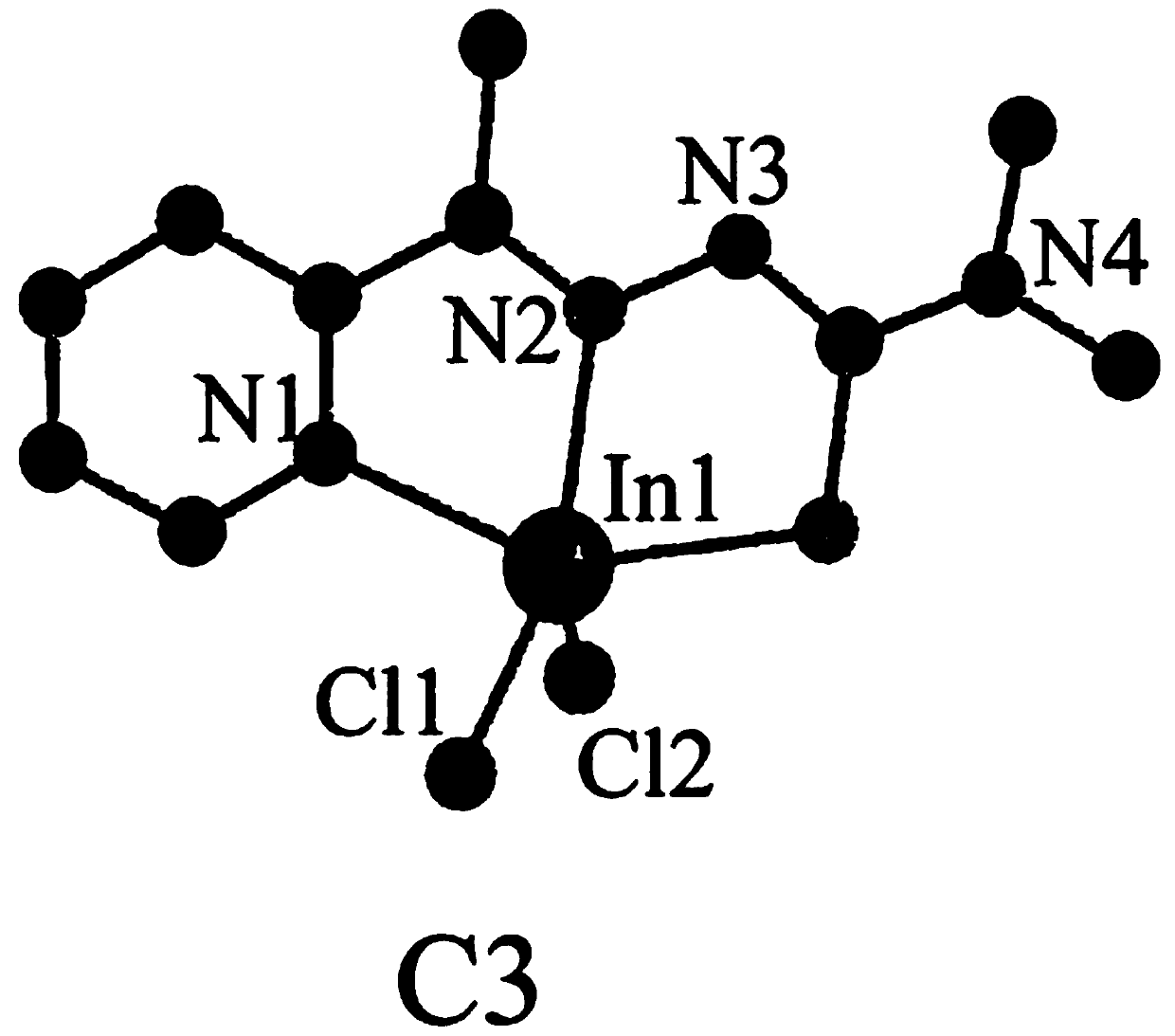
Embodiment 3
[0040] 1) Dissolve 2-acetylpyridine (363mg, 3mmol) in a mixture of 10mL methanol and 10mL acetonitrile, add 4,4-dimethylthiosemicarbazide (357mg, 3mmol), drop 3-4 drops (approx. 1mL) acetic acid, reflux at 85°C for 10h, concentrate under reduced pressure after the reaction, extract with ethyl acetate, wash with saturated sodium bicarbonate and water successively, and separate the eluent from silica gel column chromatography (the eluent is petroleum ether and ethyl acetate) The mixture, the volume ratio of petroleum ether and ethyl acetate is 10:1), to obtain ligand L3 (0.531g, 79.72%, pale yellow solid);
[0041] Yield: 0.531g, 79.72%, C 10 h 14 N 4 S: C, 54.03; H, 6.35; N, 25.20; S, 14.42. Found: C, 54.01; H, 6.36; N, 25.21; (s,NH),3155(m,aromatichydrogen),1596(m),1503(s),1458(s,aromatic),1397(m,C=N),1292(s,thioamide),1154(s) ,1105(s),883(m,C-H),714(m,C=S),593(m). 1 H NMR (400MHz, DMSO-d 6 )δ8.71(dd, J=5.5,1.3Hz,1H),8.28(td,J=7.8,1.7Hz,1H),8.09(d,J=8.1Hz,1H),7.81–7.78(m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com