Polyacid supramolecular crystal material and preparation method thereof
A technique for supramolecular crystals and heteropolyacid salts, applied in the field of polyacid supramolecular crystal materials and their preparation, can solve the problems of difficult to control production, difficult to control reaction rate, difficult to obtain crystals, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 heteropolyacid salt (NH 4 ) 3 [CrMo 6 o 24 h 6 ]·7H 2 O synthesis process
[0024] (1) Weigh 3.100g of chromium sulfate, 5.012g of ammonium molybdate and heat the 80mL aqueous solution of ammonium molybdate to boiling (oil bath heating 140°C);
[0025] (2) 20mL aqueous solution of chromium sulfate is added thereto, and the solution becomes blue-black;
[0026] (3) Cool the solution to room temperature, then evaporate in a water bath until a large amount of purple substance is precipitated, and a small amount of liquid remains in the solution (about two hours);
[0027] (4) After filtering the remaining hot solution while hot, get the filtrate and seal it with a film to prevent pollution;
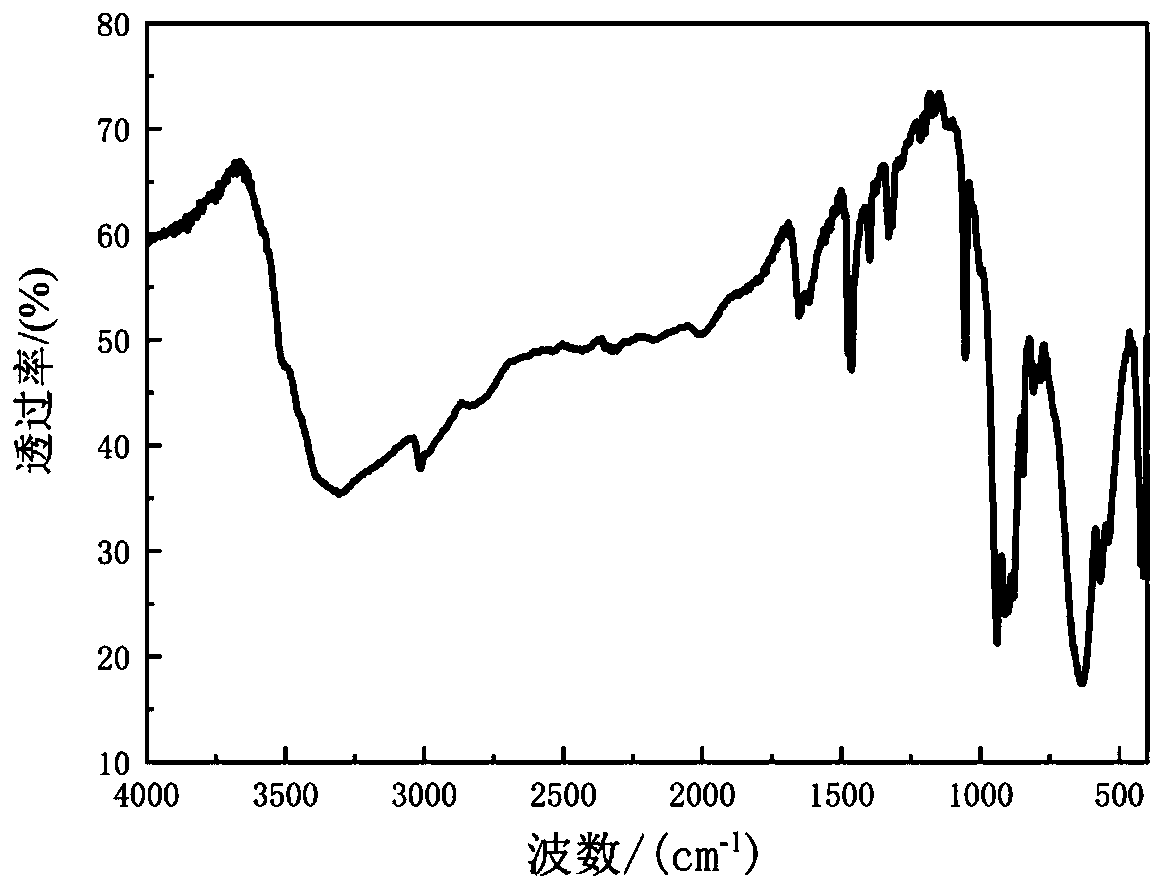
[0028] (5) The filtrate was allowed to stand for 1 to 2 days, and a small amount of liquid remained, which was filtered and dried to obtain product 1, and the weight of the yield was 3.286 g. And this product 1 carries out infrared spectrogram analysis, consistent...
Embodiment 2
[0029] Embodiment 2 (DABCO 2HBF 4 ) synthesis of salt
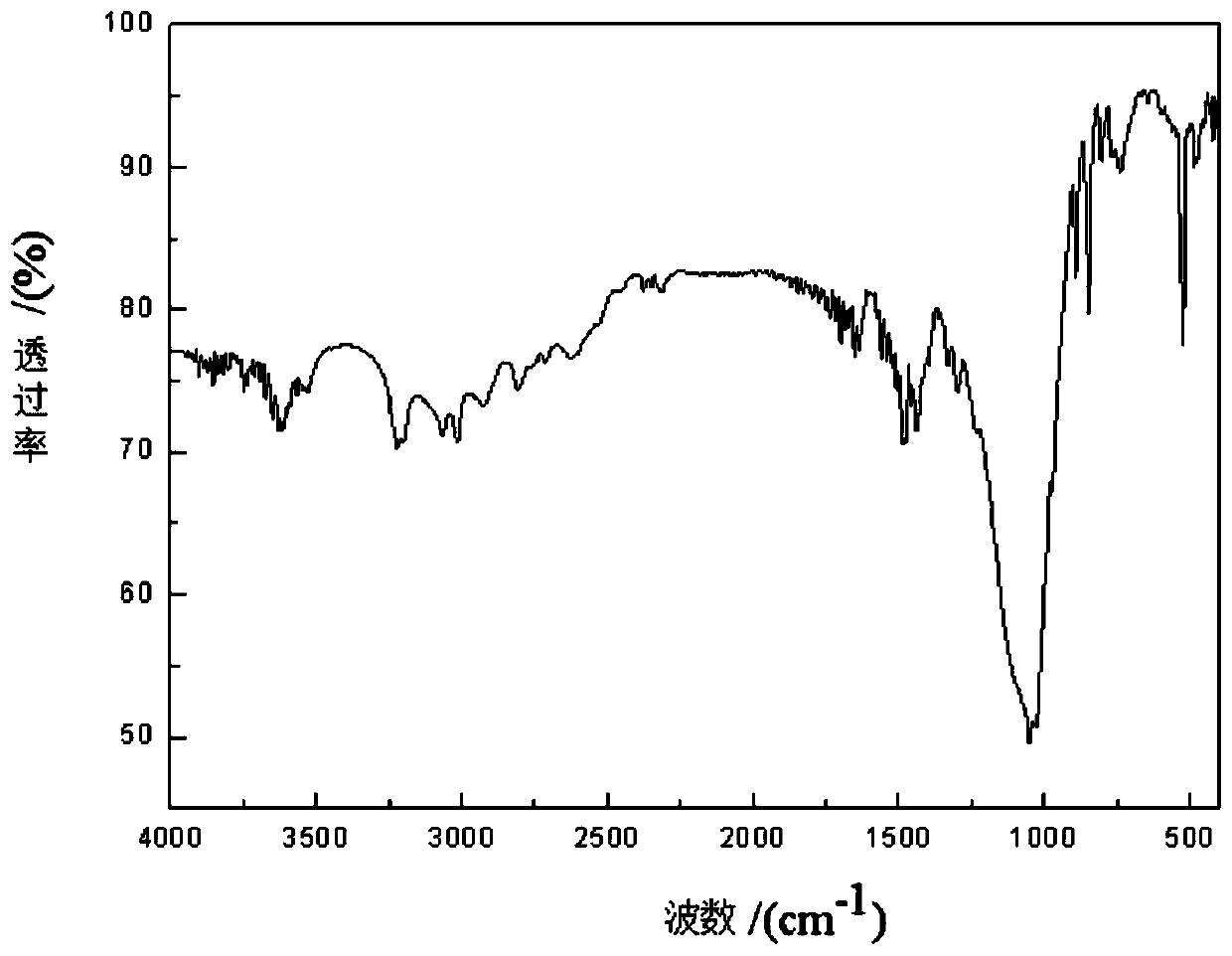
[0030] Weigh 0.500g of DABCO and dissolve it with 10mL of methanol, then weigh 1.9568g of 40% tetrafluoroboric acid and dissolve it with 10mL of methanol, drop the latter into the former solution and immediately generate a white substance, seal it with a paper towel and a rubber band, After the solvent was evaporated to dryness, the product 2 was obtained. Product 2 is carried out infrared spectrogram analysis, the result is as follows figure 1 As shown, through its infrared spectrogram analysis, it can be known that the materials required for the synthesis of raw materials all exist, and it is determined that the product 2 is the target raw material (DABCO 2HBF 4 )Salt.
Embodiment 3
[0031] The synthesis of embodiment 3 polyacid supramolecular crystal material
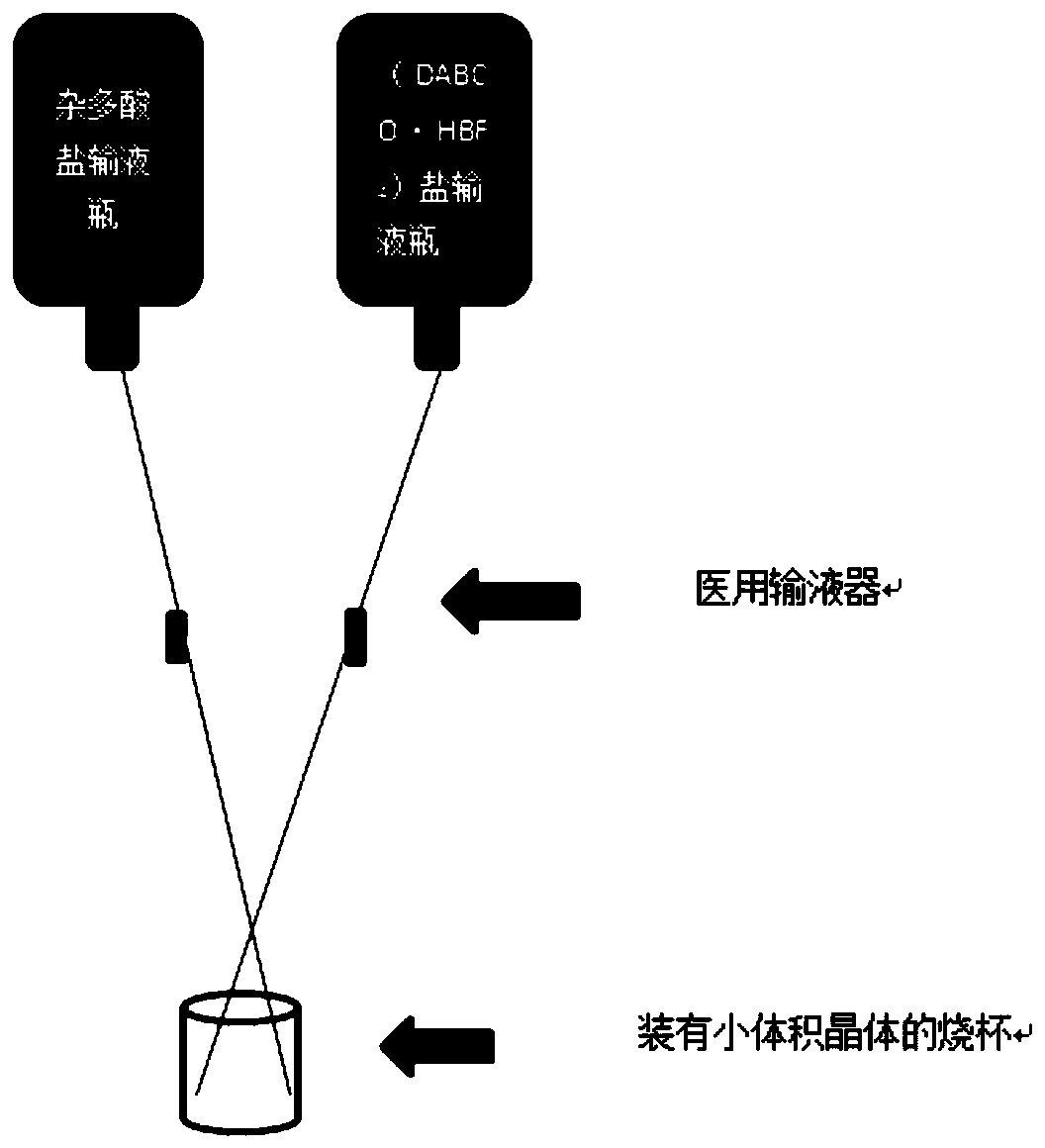
[0032] Weigh separately (NH 4 ) 3 [CrMo 6 o 24 h 6 ]·7H 2 O and (DABCO·2HBF 4 ) salt 0.020g and 0.005g, and dissolve them with 50mL of deionized water respectively. All controlled in the beaker of the solution to be replenished (such as figure 2 As shown), after the solution in the dripping bottle has been dripped for one day, use the same method to replenish the solution again, but when replenishing the solution for the second time, first absorb part of the solution in the beaker with a dropper, and the remaining solution in the beaker is about 50mL. After 5 days it was processed to afford 1 as purple rod-shaped crystals.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com