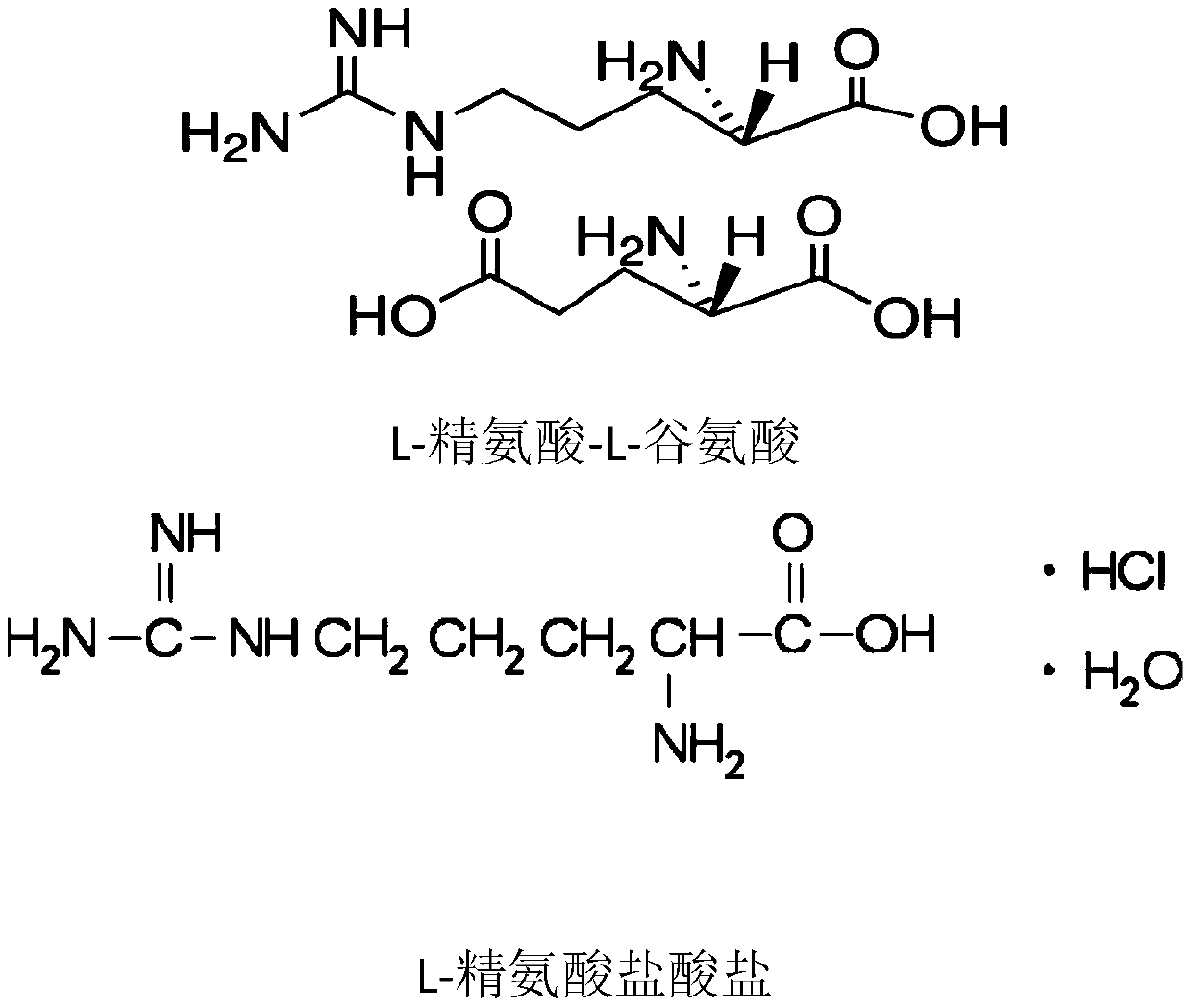
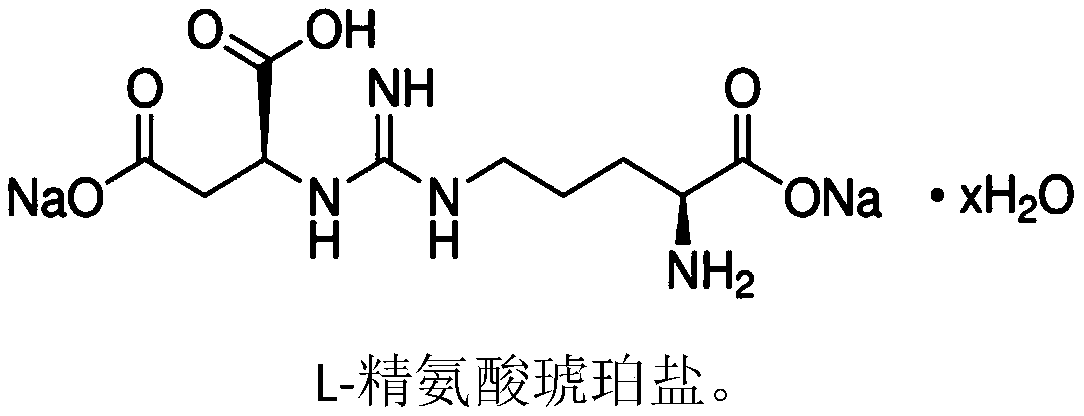
Applications of L-arginine and derivative of L-arginine in preparation of cyclododecanoneoxime, and method used for preparing cyclododecanoneoxime
A technology of arginine derivatives and cyclododecanone oxime, which is applied in the preparation of cyclododecanone oxime and the field of preparation of cyclododecanone oxime through cyclododecanone, can solve the unsolved problem of hydroxylamine stability and reaction by-products , containing some impurities such as hydroxylamine stability, long reaction time of cyclododecanone oximation, etc., to achieve the effect of eliminating the purification process, good stability and high cost performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A 1.0L glass stirred tank reactor was used as the oximation reaction vessel, and the stirred tank rotating speed was maintained at 1000rpm, and 450g Raschig solution (hydroxylamine sulfate content 12.5wt%), 190g 30wt% cyclododecanone isopropyl ring was added in advance. Hexane solution, the detected iron ion content is 158ppm, then add 15g L-arginine-L-glutamic acid as an auxiliary agent, and add ammonia water dropwise at a flow rate of 2.9g / min for 15min, the reaction temperature is controlled at 90°C, the pH Control the time between 4.5 and 6.5, use GC to track the reaction, and ICP to analyze the iron ion content in the reaction process to be 0.4ppm.
[0042] Until the conversion rate of cyclododecanone reaches more than 99.8%, the reaction time is calculated and the utilization rate of reactants is analyzed. Then add ammonia water to adjust the pH to 7.5, lower the temperature to 65°C, separate the oil and water phases, filter and recover the additives. According t...
Embodiment 2
[0044] This embodiment is with respect to embodiment 1, and difference is that the auxiliary agent that adds is the L-arginine of 15g, all the other and embodiment 1 conditions are identical, find according to analysis result, reaction time is 4.1 hours, the decomposition of hydroxylamine sulfate in the reactant The ratio is 1.2%, and the cyclododecanoneimine content is 6.5ppm (based on the total mass of the reaction solution). The conversion rate of cyclododecanone is 99.9%, the selectivity of cyclododecanone oxime is 99.992%, and the iron ion content in the reaction process is 0.9ppm.
Embodiment 3
[0046] Compared with Example 1, this example is different in that the auxiliary agent added is 15g of L-arginine succinate, and all the other conditions are the same as in Example 1. According to the analysis results, it is found that the reaction time is 2 hours, and hydroxylamine sulfate in the reactant The decomposition rate is 0.5%, and the cyclododecanone imine content is 1.6ppm (based on the total mass of the reaction solution). The conversion rate of cyclododecanone is 99.9%, the selectivity of cyclododecanone oxime is 99.998%, and the iron ion content in the reaction process is 0.1ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com