Micro-reactor tandem synthesis method of indole anticancer drug molecules
A technology of microreactors and anticancer drugs, applied in drug combinations, chemical/physical/physicochemical reactors, chemical instruments and methods, etc., can solve unseen problems, achieve mild conditions, simple production process, and side effects and the effect of fewer separation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
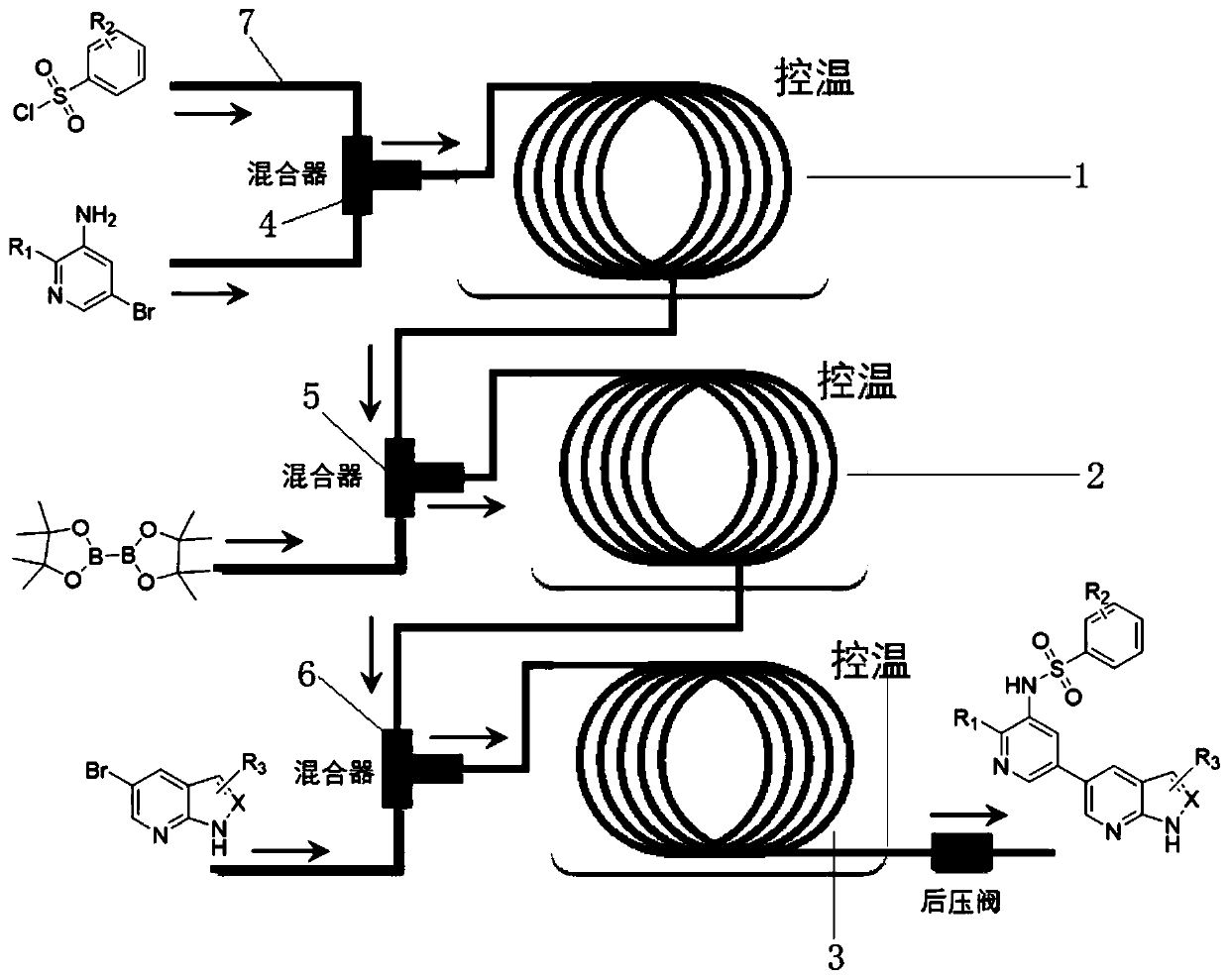
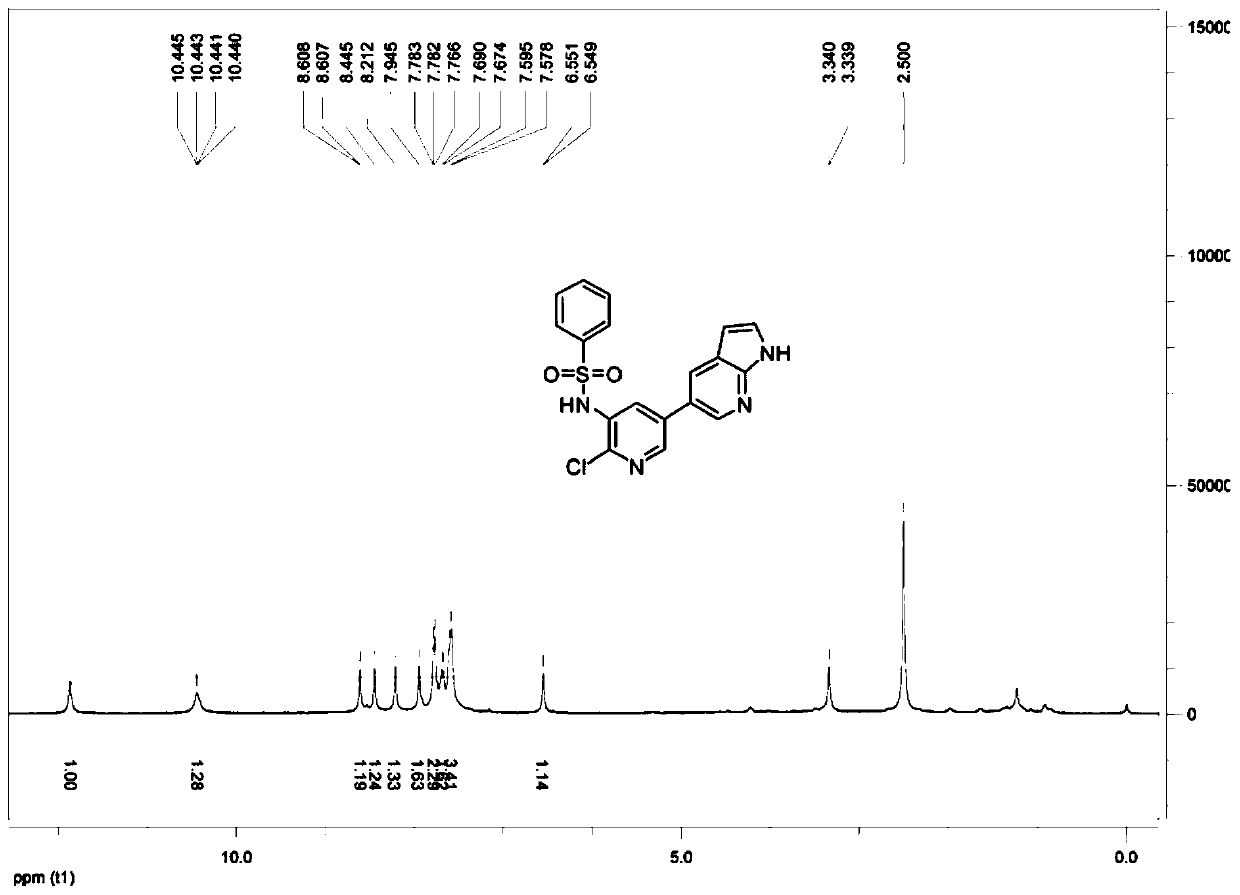
[0063] This embodiment takes N-{2-chloro-5-[1H-pyrrole(2,3-b)pyridin-5-yl]pyridin-3-yl}benzenesulfonamide as the preparation target, and the structural formula is
[0064](1) 5-bromo-3-amino-2-chloropyridine (0.10g, 0.48mmol), pyridine (0.057g, 0.72mmol), dissolved in dichloromethane (0.77mL) to prepare reaction solution 1, take Benzenesulfonyl chloride (0.10g, 0.58mmol) was made into reaction solution 2, and the flow rate (0.4mL / min) set by the intelligent numerical control injector was simultaneously introduced into the first three-way mixer (environment temperature 0°C), and then flow out under its own pressure, enter a pickle tube with an inner diameter of 500 μm at a set temperature control (25°C), and complete the sulfonamidation reaction under the set residence time t1 (1min) condition, Then through the back pressure valve, the first effluent is obtained;
[0065] (2) Dissolve bis-linked pinacolate diboron (0.18g, 0.72mmol), tetrakistriphenylphosphine palladium (0.01...
Embodiment 2
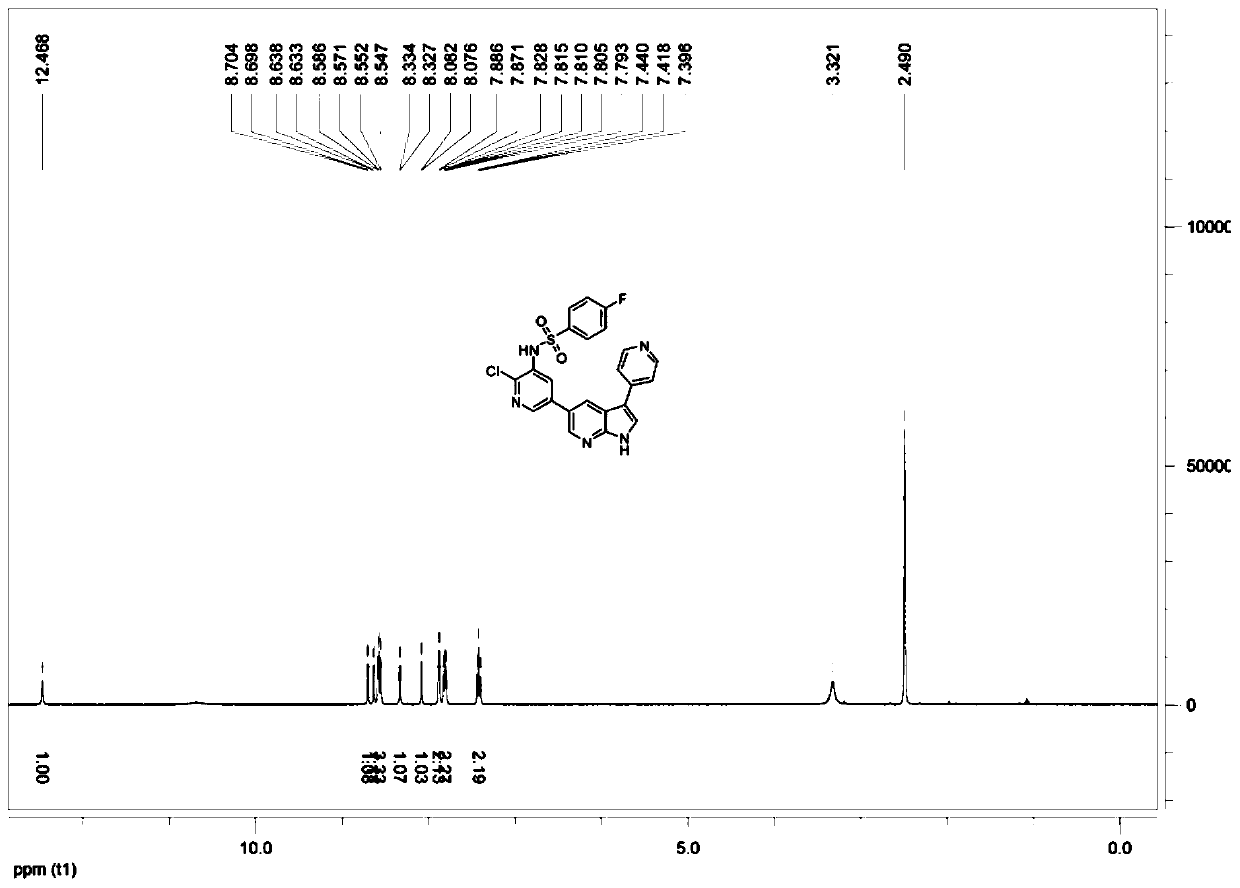
[0069] The preparation method is specifically shown in Example 1. In this example, N-{2-chloro-5-[2-methyl-1H-pyrrole (2,3-b)pyridin-5-yl]pyridin-3-yl }Benzenesulfonamide is the preparation target, and the structural formula is
[0070] (1) 5-bromo-3-amino-2-chloropyridine (0.10g, 0.48mmol), pyridine (0.057g, 0.72mmol), dissolved in dichloromethane (0.77mL) to prepare reaction solution 1, take Benzenesulfonyl chloride (0.10g, 0.58mmol) was made into reaction solution 2, and the flow rate (0.4mL / min) set by the intelligent numerical control injector was simultaneously introduced into the first three-way mixer (environment temperature 0°C), and then flow out under its own pressure, enter a pickle tube with an inner diameter of 500 μm at a set temperature control (25°C), and complete the sulfonamidation reaction under the set residence time t1 (1min) condition, Then through the back pressure valve, the first effluent is obtained;
[0071] (2) Dissolve bis-linked pinacolate dib...
Embodiment 3
[0075] The preparation method is specifically shown in Example 1. In this example, N-{2-chloro-5-[2-ethyl-1H-pyrrole (2,3-b)pyridin-5-yl]pyridin-3-yl }Benzenesulfonamide is the preparation target, and the structural formula is
[0076] (1) 5-bromo-3-amino-2-chloropyridine (0.10g, 0.48mmol), pyridine (0.076g, 0.96mmol), dissolved in dichloromethane (0.92mL) to prepare reaction solution 1, take Benzenesulfonyl chloride (0.10g, 0.58mmol) was made into reaction solution 2, and the flow rate (0.5mL / min) set by the intelligent numerical control sampler was introduced into the first three-way mixer (environment temperature 5°C), and then flow out under its own pressure, into a pickle tube with an inner diameter of 1000 μm at a set temperature control (25°C), and complete the sulfonamidation reaction under the set residence time t1 (0.5min) , and then through the back pressure valve to obtain the first effluent;
[0077] (2) Dissolve bis-pinacolyl diboron (0.18g, 0.72mmol), tetraki...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com