Preparation method for intermediate compound of iclaprim
A technology for intermediates and compounds, applied in the field of preparation of intermediate compounds, can solve problems such as expensive reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
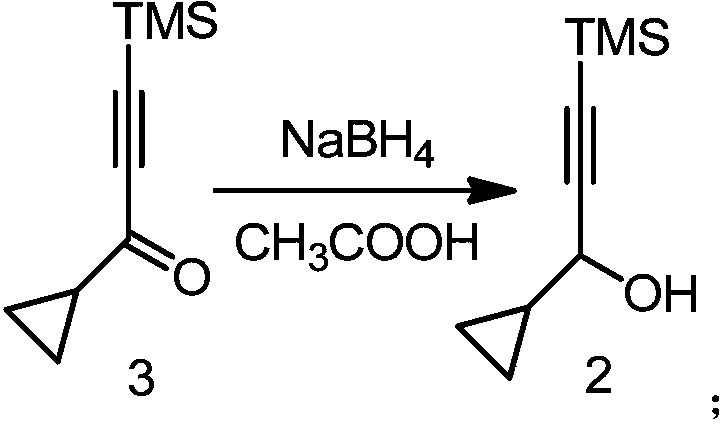
[0039] The present invention provides a kind of preparation method of the intermediate 2 of Elapulene as shown below, it comprises the following steps:
[0040]
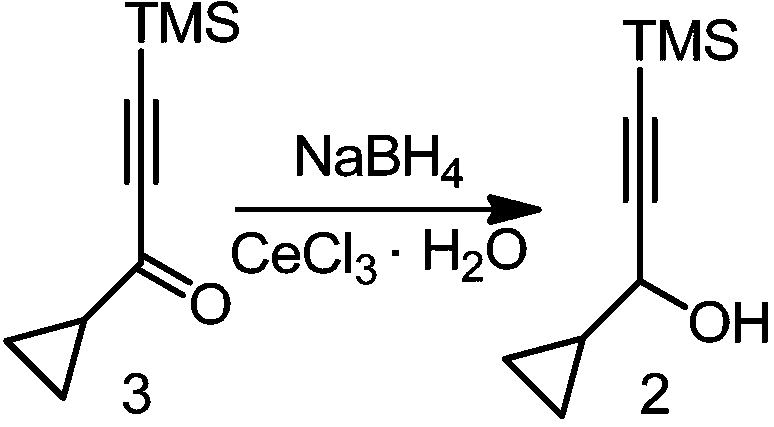
[0041] In an organic solvent, in the presence of acetic acid and sodium borohydride, intermediate 3 (i.e. 1-cyclopropyl-3-(trimethylsilyl) propyl-2-yn-1-one) and sodium borohydride A reduction reaction affords intermediate 2 (ie, 1-cyclopropyl-3-(trimethylsilyl)propyl-2-yn-1-ol).
[0042] In another preferred example, the preparation method includes the steps of:
[0043] (1) After intermediate 3 and acetic acid are dissolved in the organic solvent, sodium borohydride is added to obtain a reaction mixture;
[0044] (2) The reaction mixture obtained in step (1) is reacted at 0-50° C., and the intermediate 2 is obtained after the reaction is completed.
[0045] In another preferred example, step (2) further includes a post-processing step for the reaction mixture after the reaction, and the post-processing step is...
Embodiment 1
[0069] Example 1 Preparation of 1-cyclopropyl-3-(trimethylsilyl)propyl-2-yn-1-ol (intermediate 2)
[0070] Add 50.0 g (0.30 mol) of intermediate 3, 36.0 g of acetic acid (0.60 mol), and 500 ml of tetrahydrofuran into a 1000 ml four-necked flask, and add 11.35 g of sodium borohydride (0.30 mol) in portions under stirring. After reacting at 20° C. for 4 h, TLC (petroleum ether:ethyl acetate=20:1) showed that no raw material remained. Pour the reaction solution into 180ml of 2mol / L hydrochloric acid and dilute with 400ml of ice water. Extracted three times with 1000ml petroleum ether, washed the organic phase twice with 200ml ice water, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure to obtain 47.8g of yellow oil, and after vacuum distillation, 41.6g of colorless liquid was obtained (boiling point: 76-79°C , 5mmHg), the yield is 82.3%, and the purity is higher than 98%.
Embodiment 2
[0071] Example 2 Preparation of 1-cyclopropyl-3-(trimethylsilyl)propyl-2-yn-1-ol (intermediate 2)
[0072] Add 50.0 g (0.30 mol) of intermediate 3, 36.0 g of acetic acid (0.60 mol), and 500 ml of methanol into a 1000 ml four-necked flask, and add 11.35 g of sodium borohydride (0.30 mol) in portions under stirring. After reacting at 20° C. for 4 h, TLC (petroleum ether:ethyl acetate=20:1) showed that no raw material remained. Pour the reaction solution into 180ml of 2mol / L hydrochloric acid and dilute with 400ml of ice water. Extracted three times with 1000ml of petroleum ether, washed the organic phase twice with 200ml of ice water, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure to obtain 47.1g of yellow oil, and after vacuum distillation, 35.7g of colorless liquid was obtained (boiling point: 76-79°C , 5mmHg), the yield is 70.6%, and the purity is higher than 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com