Application of icaritin hydrate for preparing medicine capable of inhibiting STAT3 (Signal Transducer and Activator 3 of Transcription) signal pathway and preventing and curing oral carcinoma
A technology of hydrating icariin and signaling pathway, applied in the field of medicine, can solve the problem that the five-year survival rate of oral cancer patients is not significantly improved, and achieve the effects of inducing cancer cell apoptosis, inhibiting proliferation, and low biological toxicity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
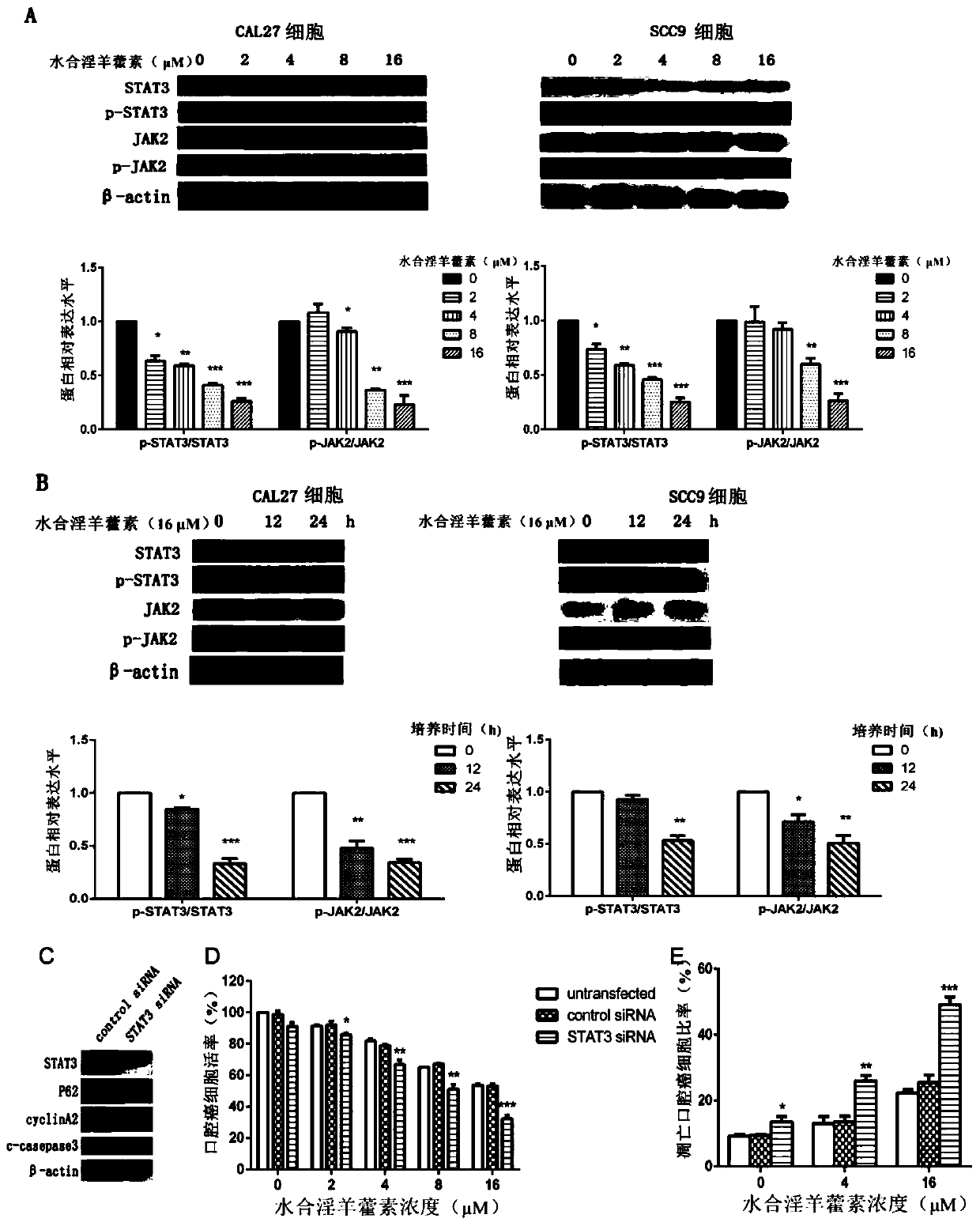
[0044] Application of icariin hydrate in inhibiting STAT3 signaling pathway in oral cancer cells.
[0045] The specific method is as follows:
[0046] Protein extraction, concentration determination and denaturation: After cultivating oral cancer cells with icariin hydrate at various concentrations (0, 2 μM, 4 μM, 8 μM, 16 μM) for a specified time, add 100 μL of lysate to a 10 cm dish, operate on ice, and collect oral cavity Cancer cell lysate. The cell lysate was then transferred to an EP tube, and protease inhibitors (lysate: PMSF = 100:1) were added. Place the EP tube in a centrifuge at 4°C and centrifuge at 12000 r.p.m. for 15 minutes; absorb the supernatant and transfer it to a new EP tube; detect the protein concentration by BCA method; dilute the protein sample according to the required concentration, generally 4 μg / μL; According to the ratio of protein sample solution:protein sample solution 1:4, add protein sample solution; heat at 96°C for 10min to denature the pro...
Embodiment 2
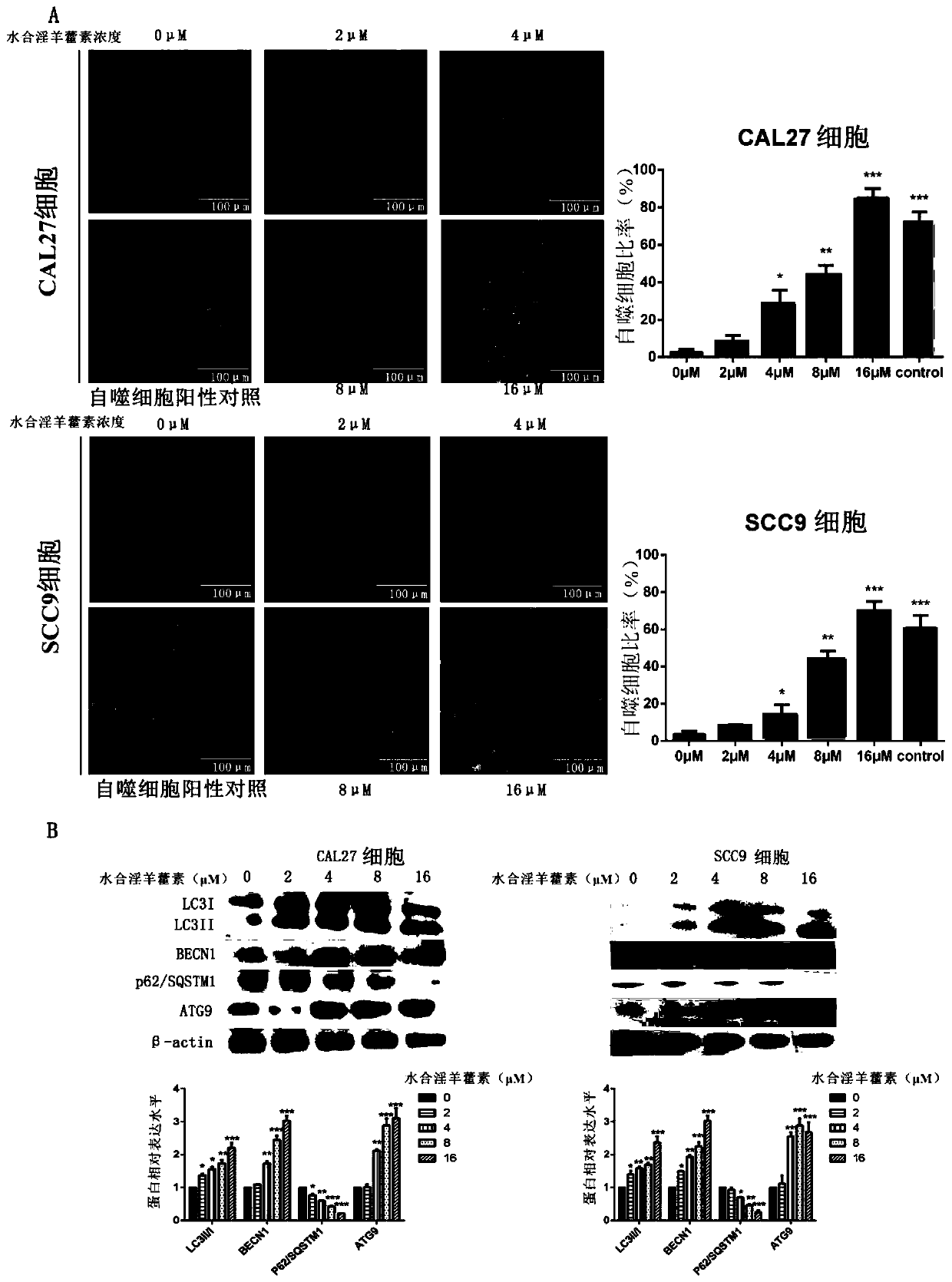
[0059] Effects of icariin hydrate on autophagy in oral cancer cells.
[0060] The specific method is as follows:
[0061] Cell autophagy MDC staining detection: 1×10 5 Oral cancer cells were inoculated in a 24-well plate with sterilized slides according to the conventional method; when the cell density reached 60%-70%, the culture medium was discarded, and the prepared concentrations (0, 2 μM, 4 μM, 8 μM, 16 μM) hydrated epimeditin for 48 hours, and 1 μM rapamycin for 3 hours as a positive control; discard the supernatant and carefully wash twice with PBS. Add 100 μL of MDC working solution (final concentration 50 nM) to each well, and incubate in the incubator for 30 minutes in the dark; discard the staining solution, wash twice with PBS, and quickly bring it into the microscope room; take out the slides, place them under a fluorescent microscope, and quickly Observe the occurrence of autophagy and take pictures.
[0062] At the same time, the effect of icariin hydrate on ...
Embodiment 3
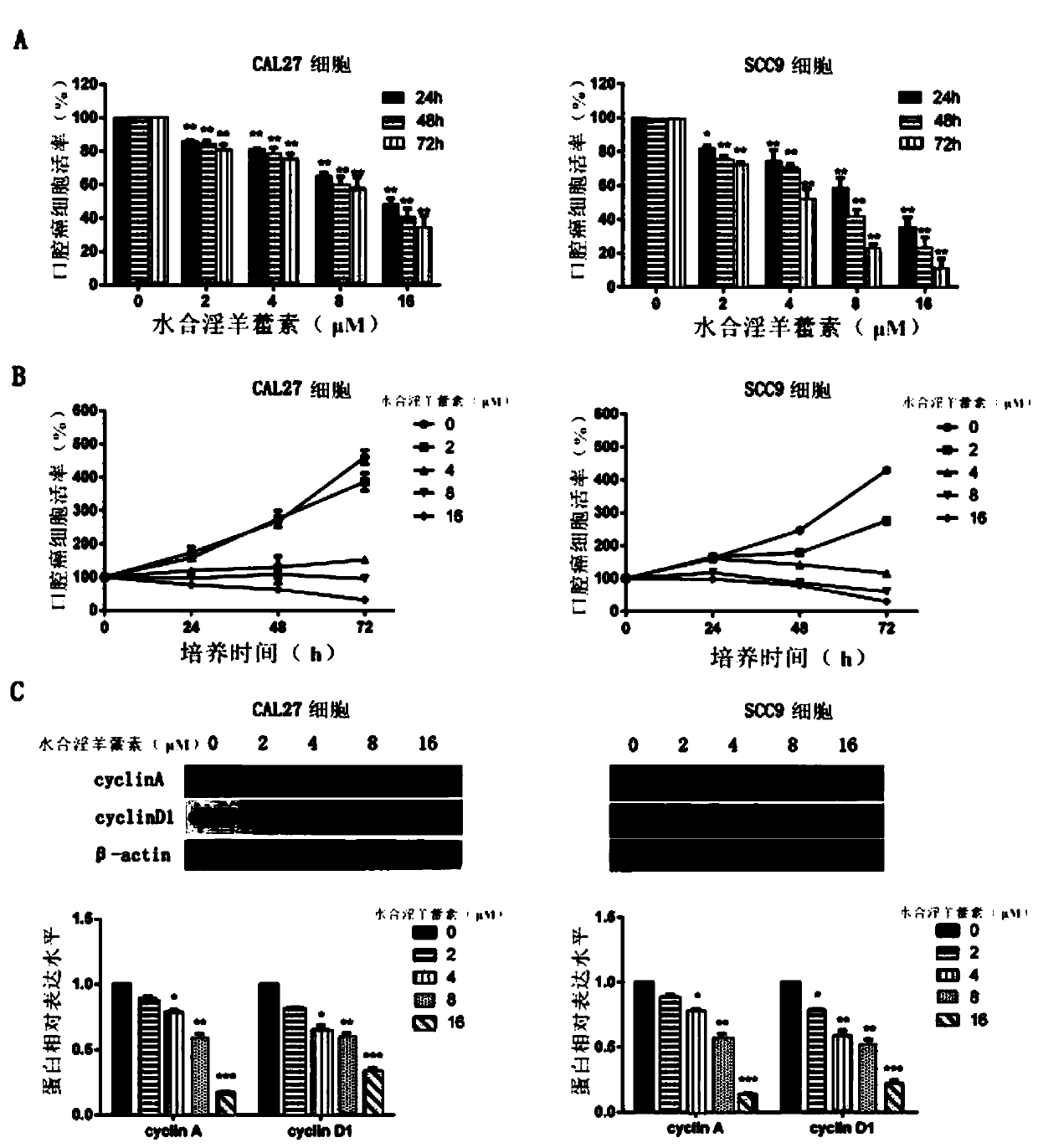
[0065] Application of icariin hydrate in the prevention and treatment of oral cancer.
[0066] In vivo experiment:
[0067] (1) To investigate the inhibitory effect of icariin hydrate on the growth of oral cancer cells.
[0068] The specific method is as follows:
[0069] Inoculate the cells of the experimental group and the control group in a 96-well plate, each well containing oral cancer cells 3×10 3 Each time period of the experimental group and the control group was set up with 6 multiple wells; put them into the incubator, at 37 ℃, 5% CO 2 Cultured for 24 hours under certain conditions to allow the cells to adhere to the wall; replace the medium with hydrated icariin at various concentrations (0, 2 μM, 4 μM, 8 μM, 16 μM); incubate the culture plate in the incubator for the specified time for 24, 48, and 72 hours ; Change 100 μL of the mixed solution in each well, that is, cell suspension + drug solution + CCK8 = 100 μL, wherein CCK8 accounts for 10%; incubate the cult...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com