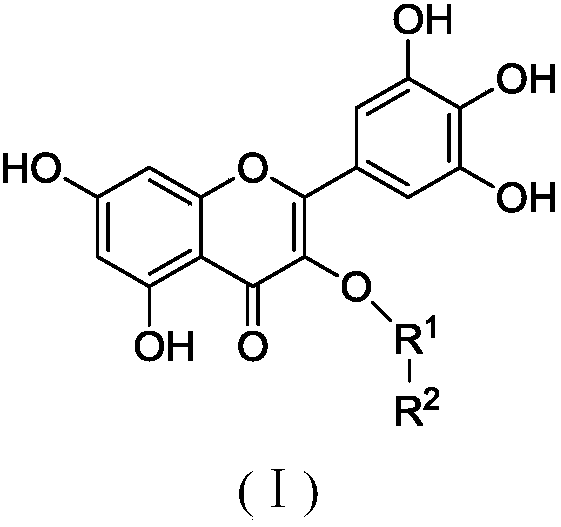
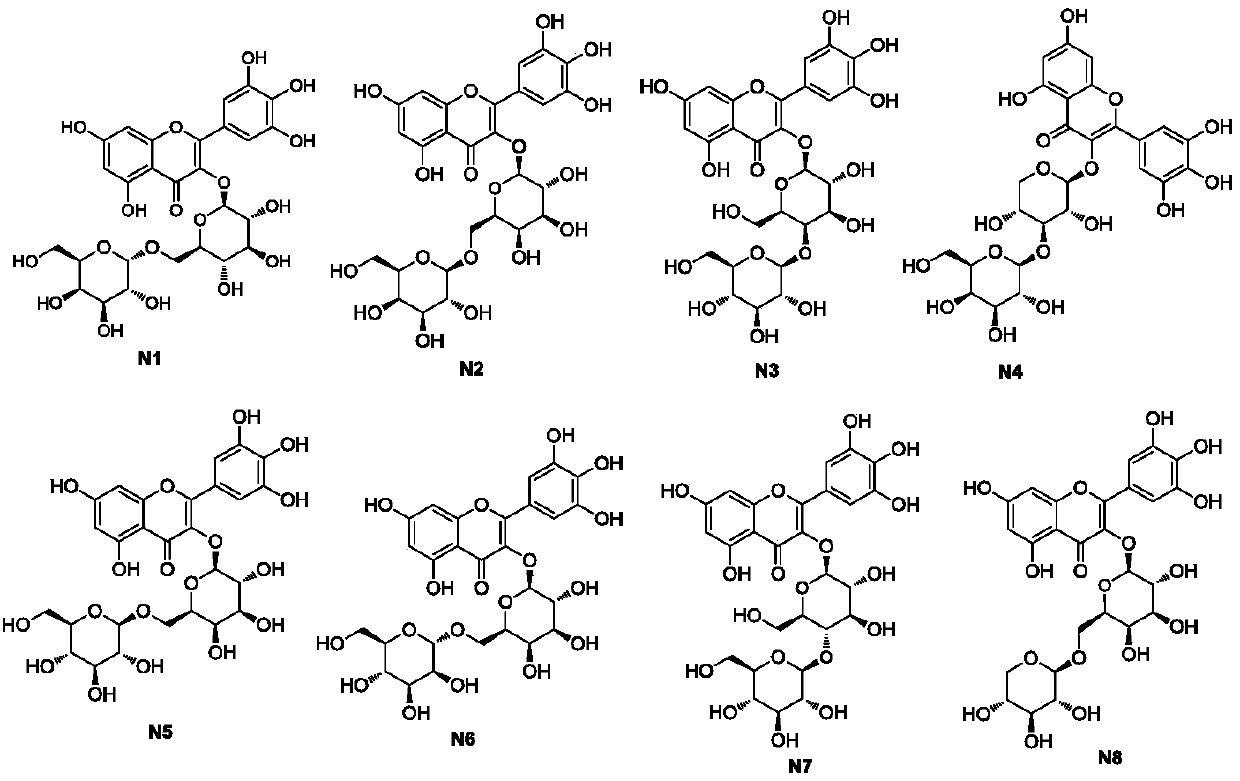
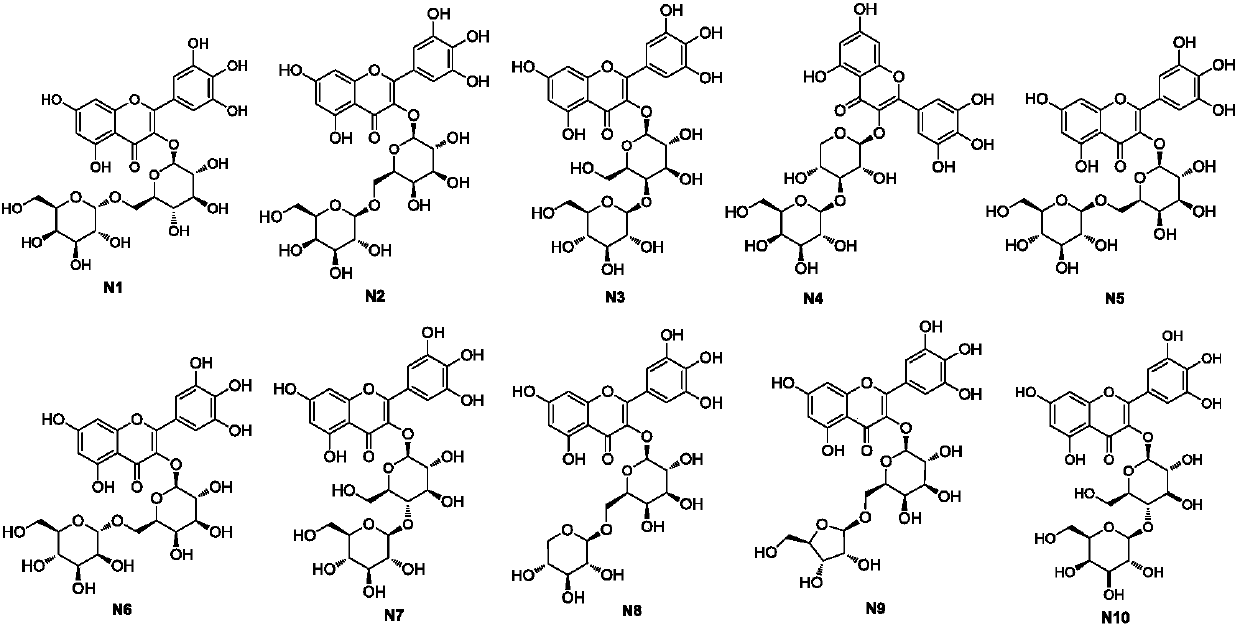
Myricetin derivative and application thereof for preparing medicine capable of reducing blood sugar and reducing blood fat
A technology of derivatives and myricetin, which is applied in the direction of sugar derivatives, drug combinations, pharmaceutical formulations, etc., can solve the problems of poor water solubility, stability and bioavailability, which affect the development and application of myricetin, and achieve a large market value, The effect of high yield and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The synthetic route of myricetin derivative M2 is as follows:
[0052]
[0053] Myricetin (100g, 0.22mol) was dissolved in N,N-dimethylformamide (1L), then potassium carbonate (300g, 2.16mol) was added, and benzyl bromide (370g, 12.16 mol), after addition, heated to 80°C for 60 hours. After the reaction was completed, cool to room temperature, add 3L of water to the reaction system, precipitate the solid under stirring, filter, add the solid to 3L of water / dichloromethane mixed solution with a volume ratio of 1:1, and adjust the pH value with 2N hydrochloric acid to Acidity, the organic phase was separated, and the aqueous phase was extracted 3 times with dichloromethane. The organic phases were combined, dried and concentrated to obtain 200 g of M2, which was directly used in the next step without purification.
Embodiment 2
[0055] The synthetic route of myricetin derivative M3 is as follows:
[0056]
[0057] Dissolve M2 (200g) obtained in Example 1 into tetrahydrofuran (1L), then add 3N hydrochloric acid (1L), and heat to reflux for 12 hours. L of ethanol / dichloromethane mixed solution with a volume ratio of 1:1 was heated to reflux for beating for 4 hours, cooled to room temperature, and filtered to obtain 67g of yellow solid M3 with a yield of 45%.
[0058] 1 H NMR (500MHz, DMSO-d6)δ=12.35(s,1H),9.85(s,1H),7.67(s,2H),7.50(t,J=6.7Hz,6H),7.39(m,12H) ,7.29(d,J=1.5Hz,2H),6.90(d,J=2.2Hz,1H),6.48(d,J=2.2Hz,1H),5.26(s,2H),5.20(s,4H) , 5.05 (s, 2H) ppm. ESI-MS: (m / z, %) = 677 [M-H]-.
Embodiment 3
[0060] The synthetic route of myricetin derivative M5-X is as follows:
[0061]
[0062] The benzyl-protected myricetin derivative M3 (30g, 44.2mmol) was added into dichloromethane (500mL), and then various acetyl-protected bromoglycosides (53mmol), tetrabutylammonium bromide (17g, 53mmol) and potassium carbonate (23g, 165.8mmol) in aqueous solution (200mL), heated to 45°C and stirred for 3 hours, then added water to separate the layers, the organic phase was washed with water and saturated brine successively, dried, and column chromatography gave yellow solid M5- X.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com