Method for synthesizing galacto-N-biose
A lactose and galactose technology, applied in biochemical equipment and methods, glycosylase, glycosyltransferase, etc., achieves the effects of high practical value, easy availability of raw materials and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
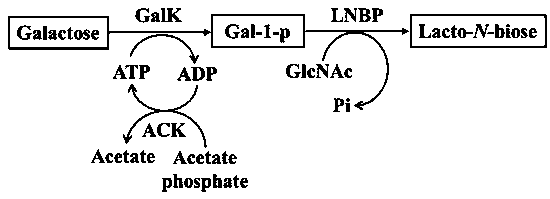
[0053] Embodiment 1: take galactose as substrate synthetic milk- N - disaccharide
[0054] Add 10 mM galactose, 10 mM ATP, 10 mM GlcNAc, 3 mM MgCl to 1 mL reaction system 2 , 100 mMTris-HCl, 5 U Galk, 168 U milk- N - Disaccharide phospholases. As shown in Table 1, wherein Control is no milk- N - Disaccharide phospholyase (LNBP) control group, after reacting at 37°C for 12 hours, the reaction system was boiled for 5 minutes, centrifuged to take the supernatant, after filtering the membrane, Biorad-HPX column, RID detection of corresponding substrates and products, reaction detection Spectrum (HPLC) comparison results verify that milk- N - Disaccharides.
[0055] Gal mM ATP mM GalK mg MgCl 2 mM
Embodiment 2
[0056] Example 2: Introduction of ATP regeneration cycle synthetic milk - N - disaccharide
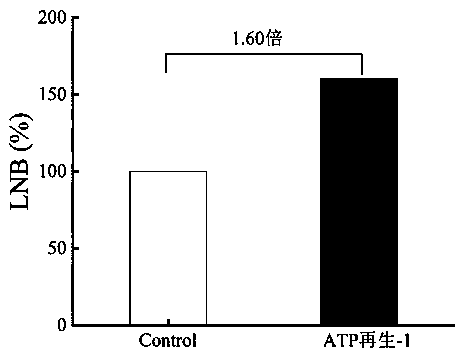
[0057] Add 10 mM galactose, 7.5 mM ATP, 2.5 mM acetyl phosphate, 10 mM GlcNAc, 3 mM MgCl to 1 mL reaction system 2 , 100 mM Tris-HCl, 5 U Galk, 3 U ACK, 168 U LNBP. As shown in Table 2, the initial concentration of ATP in the Control control was 10 mM, and the ATP cycle reaction was not introduced into the reaction system. After reacting at 37°C for 12 h, the reaction system was boiled for 5 min, and the supernatant was obtained by centrifugation. After filtering the membrane, the Biorad-HPX column , RID detects the corresponding substrate and product. Such as figure 2 As shown, under the ATP-1 reaction condition, the synthesis of LNB was increased by 1.60 times compared with the control group (without adding ATP).
Embodiment 3
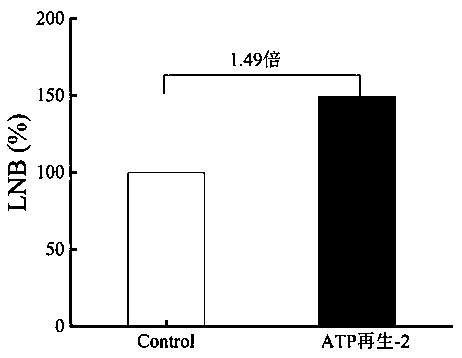
[0058] Example 3: Add 10 mM galactose, 5 mM ATP, 5 mM acetyl phosphate, 10 mM GlcNAc, 3 mM MgCl to 1 mL reaction system 2 , 100 mM Tris-HCl, 5 U Galk, 3 U ACK, 168 U LNBP. As shown in Table 2, the initial concentration of ATP in the Control control was 10 mM, and the ATP cycle reaction was not introduced into the reaction system. After reacting at 37°C for 12 h, the reaction system was boiled for 5 min, and the supernatant was collected by centrifugation. After filtering the membrane, Biorad-HPX Column, RID detects the corresponding substrate and product. Such as image 3 As shown, under the ATP-2 reaction condition, the synthesis of LNB was increased by 1.49 times compared with the control group (without adding ATP).
[0059] Table 2 The reaction table of the two-step catalyzed production of lacto-N-disaccharide with galactose substrate after the introduction of ATP regeneration cycle
[0060]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com