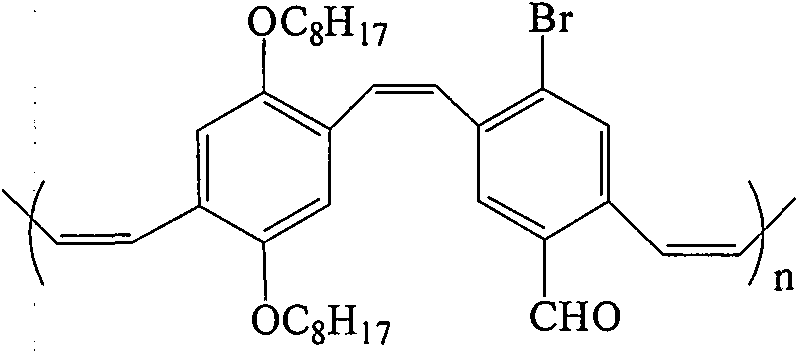
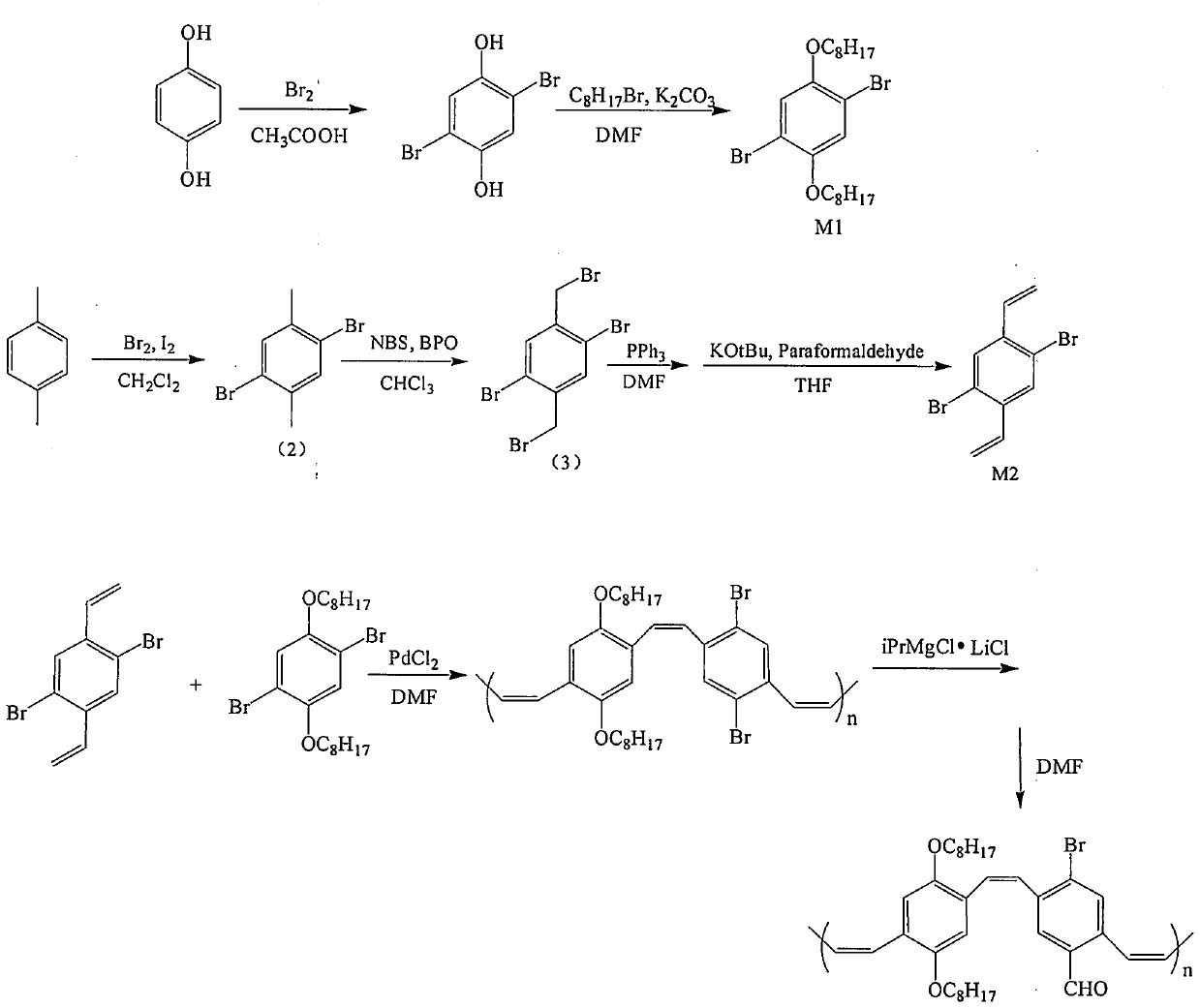
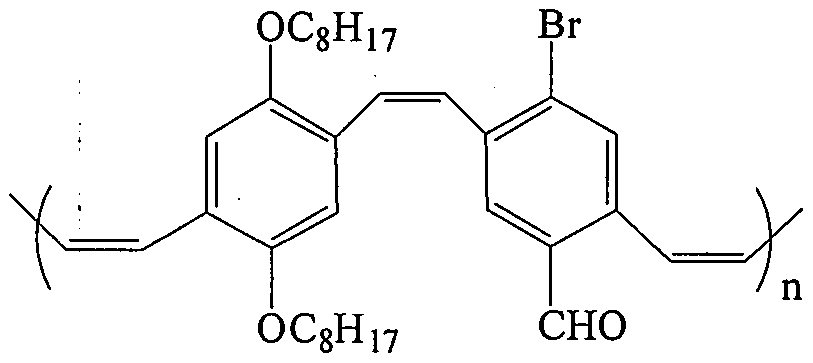
Pure organic room-temperature phosphorescence material p-bromo aryl aldehyde substituted PPV derivative and preparation method thereof
A technology of room temperature phosphorescence and derivatives, applied in the direction of luminescent materials, chemical instruments and methods, etc., can solve problems such as difficulty in realizing room temperature phosphorescence and affecting the use of materials, and achieve the effects of reducing preparation costs, easy film formation, and good photoelectric performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1) Preparation of 2,5-dibromo-1,4-benzenediol
[0022] Add 5g of hydroquinone and 50mL of glacial acetic acid in a 150mL three-necked bottle, stir and dissolve at room temperature, then add the acetic acid solution of liquid bromine (10g) drop by drop, continue to stir and react overnight at room temperature, and finally the system is cloudy. After the solid was obtained by suction filtration, the solid product was washed with a large amount of water and dried to obtain 2,5-dibromo-1,4-benzenediol.
[0023] 2) Preparation of 2,5-dibromo-1,4-dialkoxyoctylbenzene
[0024] Add 3.1g of 2,5-dibromo-1,4-benzenediol and 3.4g of potassium carbonate in a 150mL three-necked flask, dissolve them in 60mL of DMF, then add 4g of bromooctane, fill with nitrogen for protection, and heat up to 70°C, stirred and reacted for 24 hours. After the reaction was completed, the reaction mixture was naturally cooled to room temperature, and the mixture was poured into a large amount of deionize...
Embodiment 2
[0032] 1) Preparation of 2,5-dibromo-1,4-benzenediol
[0033] Add 5g of hydroquinone and 60mL of glacial acetic acid in a 150mL three-necked bottle, stir and dissolve at room temperature, then add the acetic acid solution of liquid bromine (8g) drop by drop, continue to stir and react overnight at room temperature, and finally the system is cloudy. After the solid was obtained by suction filtration, the solid product was washed with a large amount of water and dried to obtain 2,5-dibromo-1,4-benzenediol.
[0034] 2) Preparation of 2,5-dibromo-1,4-dialkoxyoctylbenzene
[0035] Add 3.1g of 2,5-dibromo-1,4-benzenediol and 3.6g of potassium carbonate to a 150mL three-necked flask, dissolve them in 60mL of DMF, then add 4.65g of bromooctane, fill with nitrogen for protection, and raise the temperature to 80°C, stirred and reacted for 20 h. After the reaction was completed, the reaction mixture was naturally cooled to room temperature, and the mixture was poured into a large amount...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Luminous life | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com