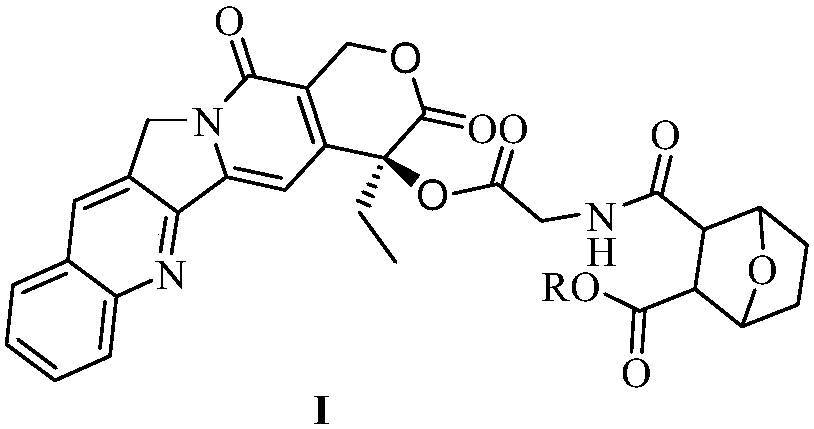
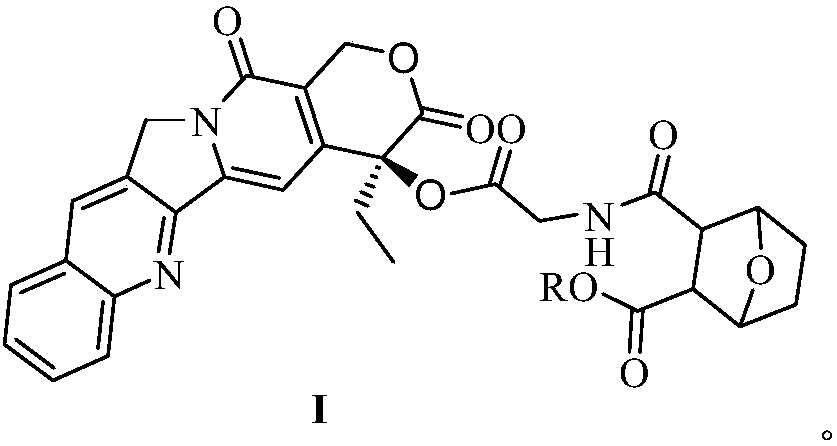
Camptothecin-glycine-norcantharidin conjugate and application thereof
A technology of norcantharidin and glycine is applied in the directions of drug combination, bulk chemical production, organic chemistry, etc., to achieve the effects of high activity, good anti-tumor effect and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1, preparation compound II
[0021] Referring to the literature, the reaction of furan and maleic anhydride in tetrahydrofuran gives 5,6-didehydronorcantharidin 4, 2), and compound 4 gets norcantharidin by catalytic hydrogenation (such as Pd / C) in tetrahydrofuran 5, 3), compound 5 is subjected to acid anhydride hydrolysis and ring-opening reaction in alcohol reagent ROH to obtain norcantharidic acid monoester II.
[0022]
[0023] R is selected from C 1 -C 6 Alkyl, benzyl, substituted benzyl
[0024] (1), preparation of 5,6-didehydronorcantharidin 4
[0025] Place 12.021 g of maleic anhydride in a dry grinder and grind finely, dissolve in 90 mL of ether, and slowly add 13 mL of furan dropwise. After the reaction solution was reacted at 38°C for 1 h, a white solid appeared in the solution, and the white solid increased with the reaction time. After reacting for 24 hours, suction filtration was performed to obtain the target compound 4 (17.459 g, 85....
Embodiment 2
[0032] Embodiment 2, preparation camptothecin-glycine-norcantharidin conjugate I
[0033] Camptothecin-glycine-norcantharidin conjugate I can be prepared by the following steps: 1), camptothecin and N-Boc-glycine 1 are obtained by esterification reaction in the presence of a coupling agent and an organic base to obtain compound 2; 2), compound 2 is removed Boc protecting group under the catalysis of trifluoroacetic acid to obtain compound 3; 3), and norcantharidin monoester II is obtained camptothecin by esterification in the presence of a coupling agent and an organic base - Glycine-norcantharidin conjugate I.
[0034] Preparation of Compound 2 (BOC-Gly-CPT)
[0035] CPT (160 mg, 0.46 mmol), N-Boc-glycine (1, 160 mg, 0.92 mmol, 2 equ.) and DMAP (0.053 g, 0.45 mmol, 1.0 equ.) were dissolved in CH 2 Cl 2 (20mL). The reaction mixture was cooled to 0 °C. DIC (0.52 mL, 3.35 mmol, 1.3 equ.) was added dropwise to the reaction mixture. The reaction was kept in an ice bath a...
Embodiment 3
[0044] Embodiment 3. Solubility experiment
[0045] Select the synthesized compound Ia and the parent compound camptothecin CPT, dissolve in chloroform at 25°C, and the solubility results are listed in Table 1.
[0046] Table 1. The solubility of compound Ia and CPT in chloroform at 25°C
[0047]
[0048] Compound Ia is many times more soluble in chloroform than CPT. It can be seen that the solubility of synthetic product I in organic solvents is much better than that of CPT.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com