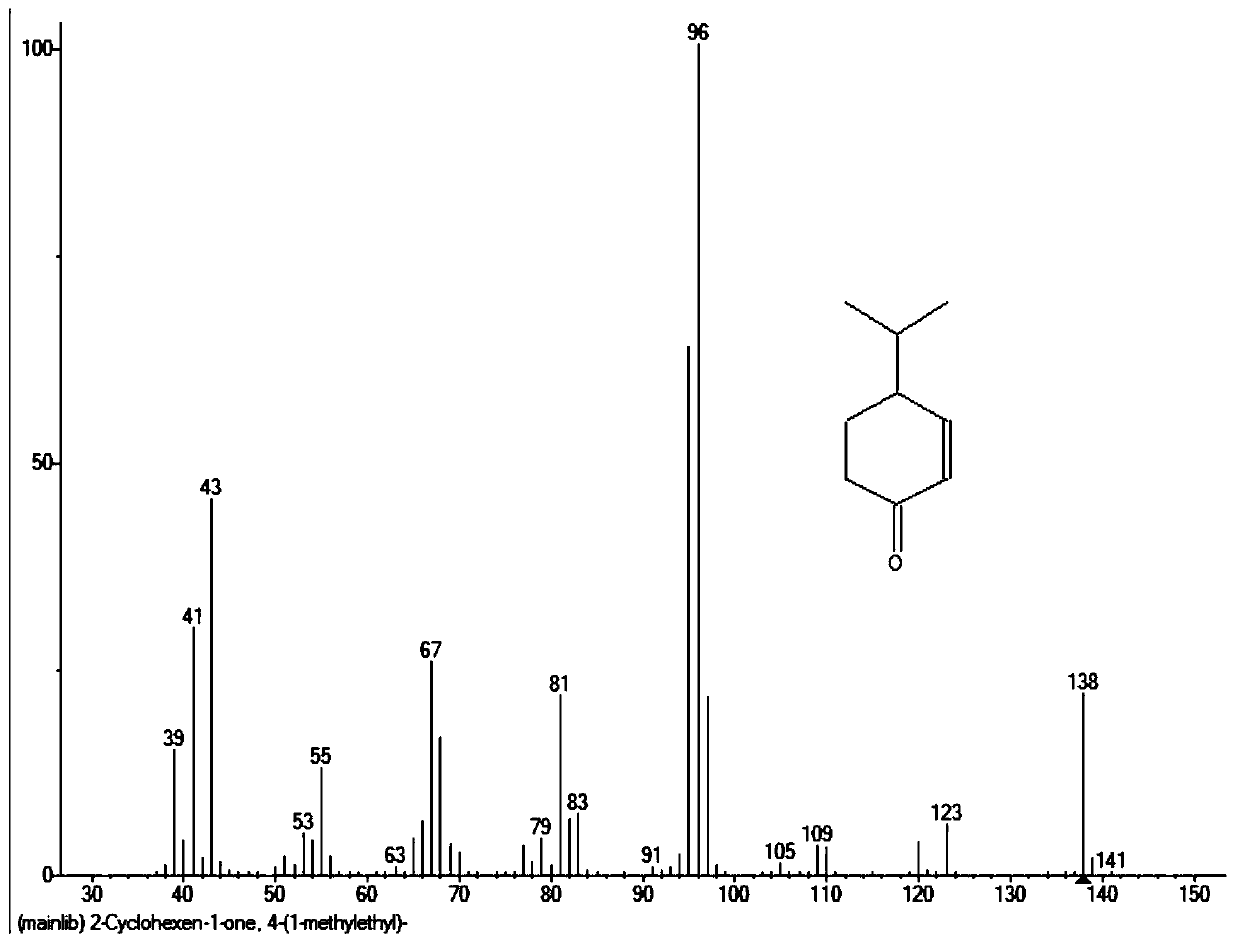
Cryptone synthesis method
A synthetic method and technology of cryptonone, which is applied in the field of synthesis of cryptonone, can solve the problems of large water pollution, difficult recovery of hexahydropyridine, poor separation of hexahydropyridine, etc., and achieve short process, easy recovery, and shortened reaction time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0033] Add 80g of tetrahydropyrrole and 170g of MTBE into a 1000ml three-necked flask, cool down to -6°C and start to add 90g of isovaleraldehyde dropwise for 2 hours. After the dropwise addition, continue to stir (normal temperature) for 2 hours, then pour into a separatory funnel to separate the water layer, washed once with 80g of water, then cooled to -4°C and started to add 66g of butenone dropwise, controlled the temperature at -4°C and added dropwise for 3h, continued to stir for 2h after the dropwise addition, and then (normal temperature 35°C) stirred for 24h after the reaction was completed Lower the temperature and keep it at 5°C, acidify with dilute sulfuric acid to adjust pH = 6, separate the water layer, wash the organic phase twice with 200 g water, the solution pH = 6-7, and recover tetrahydropyrrole with water. Add 300g of water and 2g of sodium hydroxide to the organic phase and raise the temperature to reflux, react for 4h, stop the reaction, add a separatory...
example 2
[0044] Add 70g of tetrahydropyrrole and 200g of n-hexane into a 1000ml three-necked flask, cool down to -8°C, start to add 82g of isovaleraldehyde dropwise and react for 4h, then pour into a separatory funnel to separate the water layer, and wash once with 70g of water. Then lower the temperature to -5°C and start adding 60g of butenone dropwise, keep the temperature at -5°C and add dropwise and stir for 5h, then stir at room temperature for 24h. After the reaction is completed, then cool down to 8°C and acidify with dilute sulfuric acid to adjust ph=6, and separate the water layer, and the organic phase was washed twice with 175g water, and the water reclaimed tetrahydropyrrole. Add 275g of water and 1.8g of sodium hydroxide to the organic phase and raise the temperature to reflux, react for 4h, and stop the reaction. Add a separatory funnel to separate the water layer. The oil layer was recovered and normal pressure distilled 70-85°C n-hexane, and then distilled under reduce...
example 3
[0046] Add 77.8g of tetrahydropyrrole and 240g of cyclohexane into a 1000ml three-neck flask, cool down to -5°C and start adding 82g of isovaleraldehyde dropwise for 3 hours, then pour into a separatory funnel to separate the water layer, and wash once with 78g of water. Then lower the temperature to -3°C and start adding 55.6g of butenone dropwise, control and maintain the temperature at -3°C and add dropwise and stir for 6h, then stir at room temperature for 30h. The aqueous layer was taken out, and the organic phase was washed twice with 220 g of water, and tetrahydropyrrole was recovered from the water. Add 260g of water and 1.7g of sodium hydroxide to the organic phase and heat up to reflux, react for 4h, stop the reaction, add a separatory funnel to separate the water layer. The oil layer was recovered and 180g of cyclohexane was distilled at 80-90°C under atmospheric pressure, and then distilled under reduced pressure to obtain the crude cryptonone, and after rectificat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com