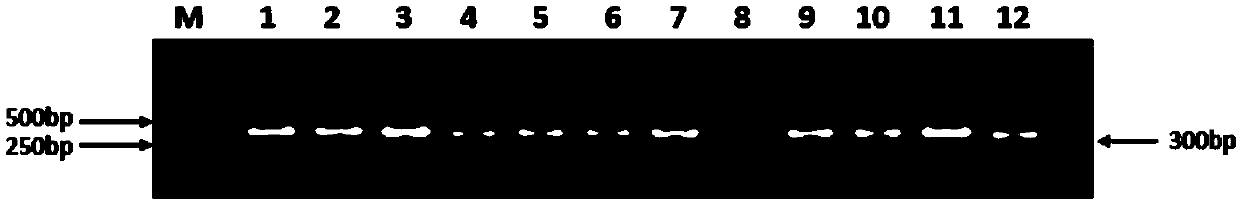
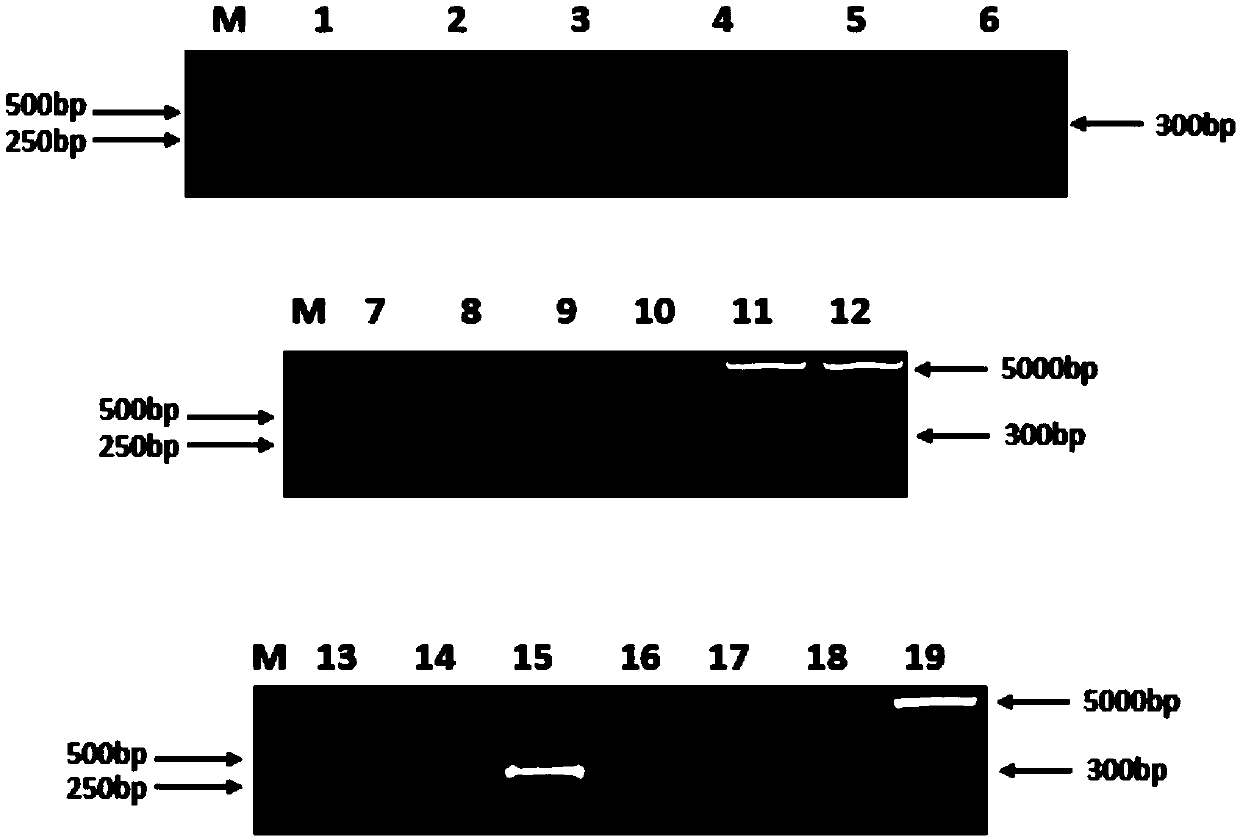
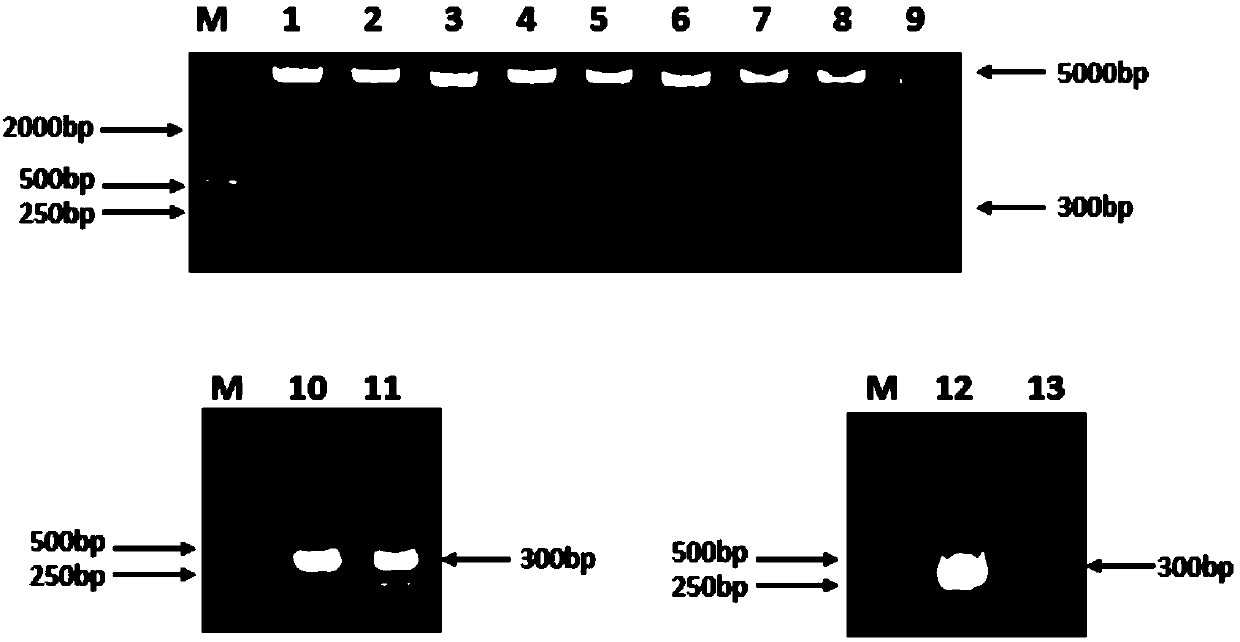
Fully human monoclonal antibody for neutralizing enterovirus 71 (EV71) and application of fully human monoclonal antibody
A monoclonal antibody and enterovirus technology, applied in the field of biological immunology, can solve problems such as adverse reactions, lack of anti-EV71 drugs, inability to prevent EV71 virus subtypes, etc., and achieve good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Preparation of a fully human monoclonal antibody neutralizing enterovirus 71
[0054] 1. Sorting of memory B cells
[0055] 1.1 Acquisition of peripheral blood mononuclear cells
[0056] 3.5ml of fresh blood was drawn from volunteers infected with enterovirus 71 and placed in an anticoagulant tube. After diluting the fresh anticoagulated whole blood with PBS in an equal volume, slowly add it to the lymphocyte separation medium (domestic Solarbio, Cat.NO.P8610), take it out carefully after centrifugation, and absorb the second layer of cloudy monocytes The layer was washed in a centrifuge tube filled with PBS, the supernatant was discarded by centrifugation, and then washed once with PBS, and the collected cells were peripheral blood mononuclear cells (PBMC).
[0057] 1.2 Memory B cell sorting
[0058] Centrifuge the isolated PBMCs from volunteers to remove the supernatant thoroughly, every 10 7 Resuspend the cell pellet with 40 μL buffer. Add 10μL Biotin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com