Application of Zearalenone Degrading Enzyme in Hydrolyzing Zearalenone and Its Derivatives
A technology of zearalenone and zearalenol is applied in the application field of zearalenone degrading enzyme in hydrolyzing zearalenone and its derivatives, and can solve the problem of zearalenone degrading enzyme Problems such as poor stability and narrow PH range, to achieve the effects of excellent thermal stability, good stability and outstanding thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
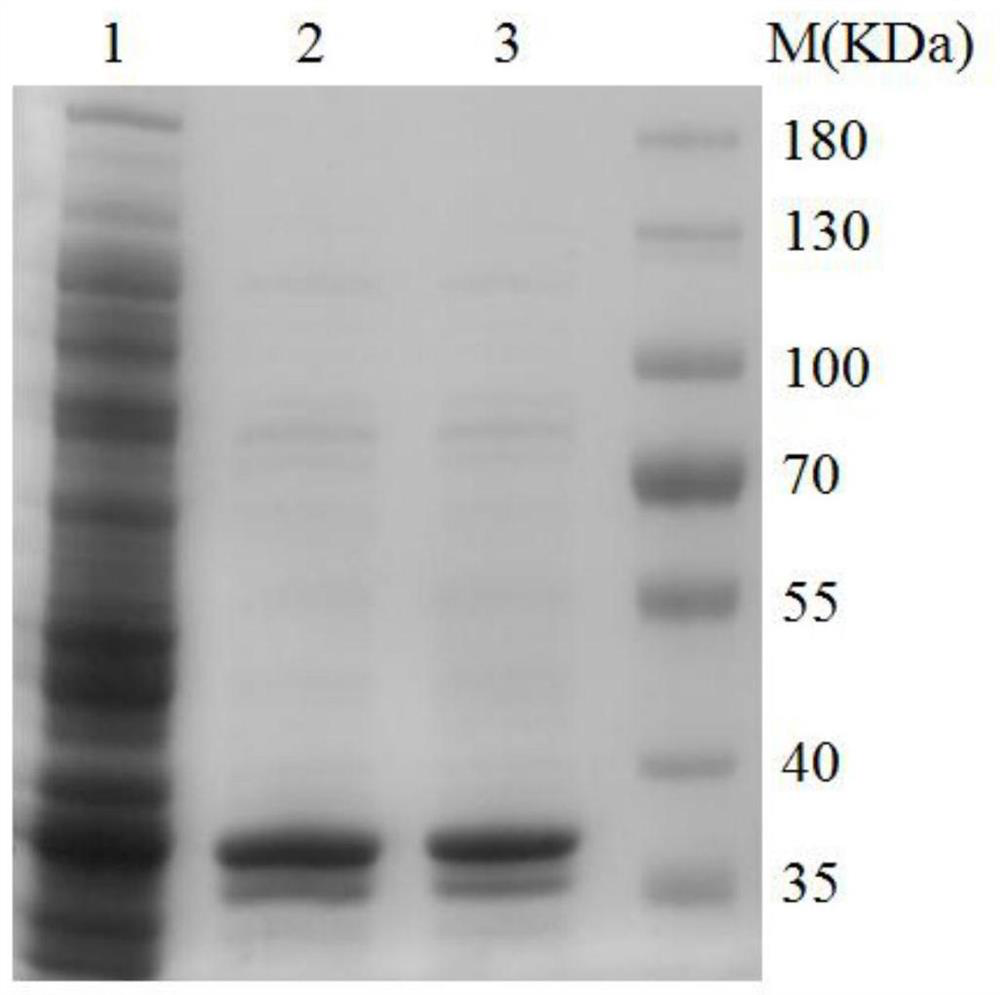
[0030] Example 1 Preparation and Purification of Zearalenone Degrading Enzyme
[0031] (1) Artificial synthesis of gene sequence
[0032] Entrust Wuhan Jinkairui Bioengineering Co., Ltd. to synthesize the nucleotide sequence shown in SEQ ID NO: 2, and insert the sequence into the plasmid vector pET26b, and save it for future use.
[0033] The sequence of SEQ ID NO:2 is as follows:
[0034] atgtccgccgagagagttagatccaccgttttgactaaggacggtatcaactggtactacgagcaagaaggtactggtccagacttggttttgatcccagatggtttgggtgactgccagatgttcgataagccaatgtccttgattgcctcctccagattcaaggttaccaccttcgatatgccaggtatgtccagatcttctgctgctccaccagaaacctaccaagaggttactggtgagaagttggctacctacatcgacaccttgatggacaagctggacatcactactgcttccgtttggggttgttcttctggtgcttctactgttttggccctgtgtgctaacttcccacacagagttagaaacgctatgccacacgagctgccaactactaatccaccatccattgacaacatccacgaagctgatccagctactttgccagctgctttggctgctactatcagaactatgtctggtggtgaagctgcttgggatgctttgggtcctgaagttcacgatagactgagagccaacaactacgttagatgggcttacggttacccaagaactattccaggttccgctccaa...
Embodiment 2
[0058] Example 2 Using zearalenone as a substrate to verify the function of zearalenone-degrading enzymes
[0059] The enzyme activity unit is defined as the amount of enzyme required to degrade 1 μg of the substrate zearalenone within 1 min as an enzyme activity unit U.
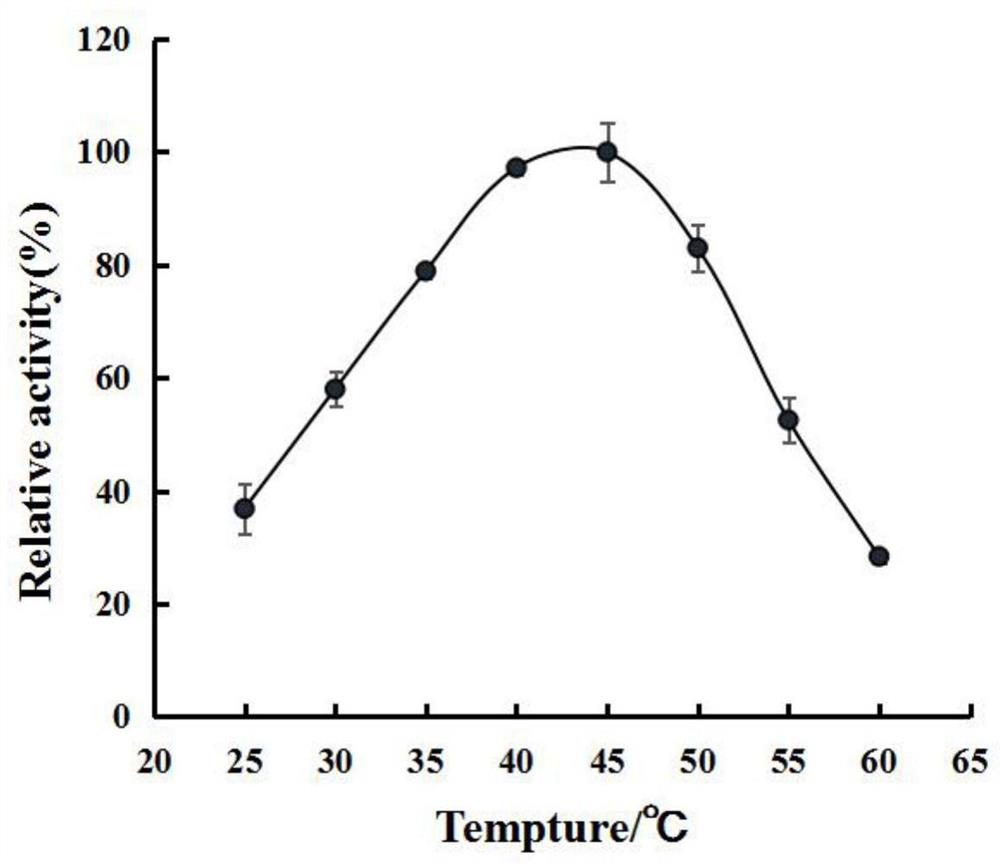
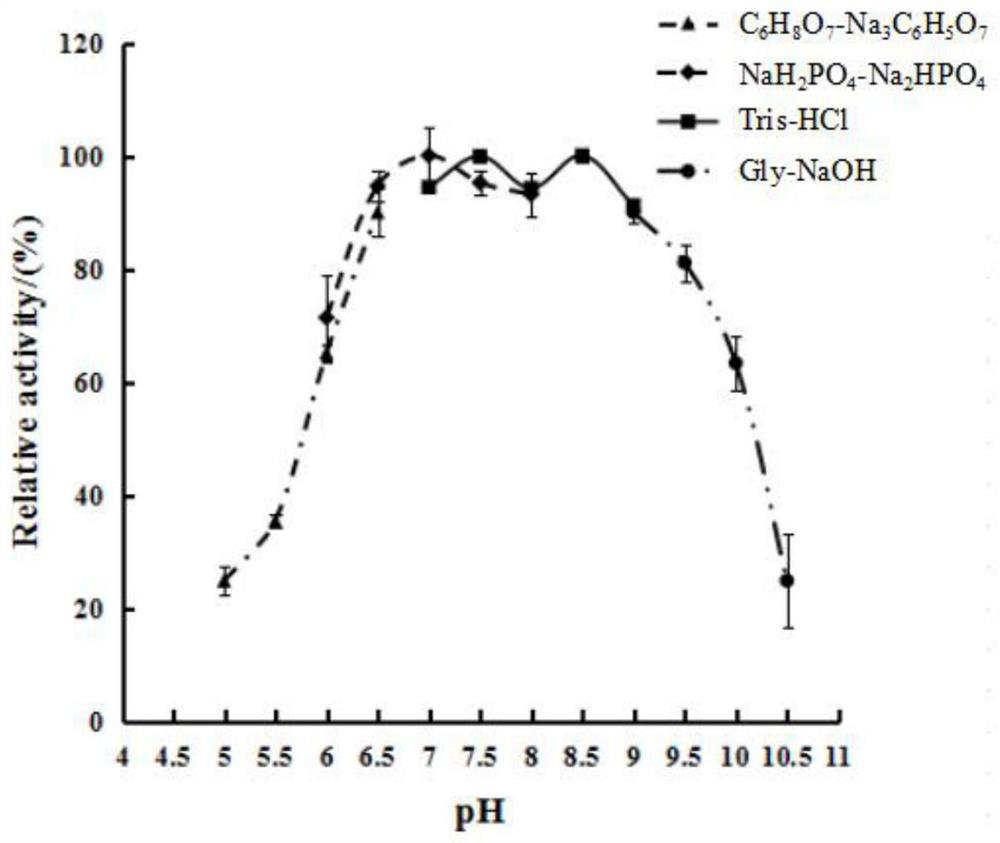
[0060] (1) Optimum temperature
[0061] The Zhd11c pure enzyme solution in Step 5 of Example 1 was diluted with 50 mM Tris-HCl buffer solution of pH 8.0, and the enzyme activity was measured with the diluted enzyme solution. The diluted enzyme solution was recorded as the diluted enzyme solution.
[0062] The composition of solution A: consists of 50 mM, pH 8.0 Tris-HCl buffer solution and zearalenone solution; the final concentration of the substrate zearalenone in the reaction system 0.5 mL is 20.0 μg / ml.
[0063] Experimental group: The reaction system for activity determination is 0.5mL, diluted with 0.45mL solution A and 0.05mL enzyme solution; the pH value of the reaction system is 8.0; mL of chroma...
Embodiment 3
[0086] Example 3 Using β-zearalenol as a substrate to verify the function of zearalenone degrading enzyme
[0087] The enzyme activity unit is defined as the amount of enzyme required to degrade 1 μg of the substrate zearalenone within 1 min as an enzyme activity unit U.
[0088] The diluted enzyme solution described below was obtained by diluting the Zhd11c pure enzyme solution in Step 5 of Example 1 with 50 mM Tris-HCl buffer solution.
[0089] Experimental group: β-zearalenol was used as the substrate (the final concentration of the substrate in the reaction system was 20.0 μg / ml), the activity assay reaction system was 0.5mL, and 0.45mL substrate solution and 0.05mL diluted enzyme solution; the pH value of the reaction system was 8.0; after the reaction system was reacted at an optimum temperature of 40° C. for 10 min, 0.5 mL of chromatographic grade methanol was used to terminate the reaction, and after cooling, the amount of degradation of the substrate was measured usin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com